Interleukin-6 (IL-6) is a major pro-inflammatory cytokine that demonstrates a robust correlation with age and body mass index (BMI) as part of the senescence-associated secretory phenotype. IL-6 cytokines also play a crucial role in metabolic homeostasis and regenerative processes primarily via the canonical STAT3 pathway. Thus, selective modulation of IL-6 signaling may offer a unique opportunity for therapeutic interventions. Our recent studies identified a novel non-canonical signaling pathway that involves prolonged activation of SRC family of kinases (SFKs) by IL-6/gp130, where genetic or pharmacological inhibition of this pathway was protective in several acute injury models. This study was designed to assess the effect of a small molecule (R159) that inhibits the non-canonical signaling in a mouse model of multimorbidity induced by chronic inflammation. Aged mice were fed a high-fat diet (HFD) to exacerbate chronic inflammation and inflammaging-related conditions, and R159 significantly decreased systemic inflammatory responses in adipose tissue and liver. R159 was protective against trabecular bone and articular cartilage loss and markedly prevented neurogenesis decline. Moreover, R159 reduced weight gain induced by HFD and increased physical activity levels. These findings suggest that selective pharmacological inhibition of SFK signaling downstream of IL6/gp130 offers a promising strategy to alleviate systemic chronic inflammation and relevant multimorbidity.
© 2024. The Author(s).
Product Citations: 40
In Scientific Reports on 28 December 2024 by Lee, Y., Tassey, J., et al.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
Epigenetic maintenance of adult neural stem cell quiescence in the mouse hippocampus via Setd1a.
In Nature Communications on 6 July 2024 by Zhao, T., Hong, Y., et al.
Quiescence, a hallmark of adult neural stem cells (NSCs), is required for maintaining the NSC pool to support life-long continuous neurogenesis in the adult dentate gyrus (DG). Whether long-lasting epigenetic modifications maintain NSC quiescence over the long term in the adult DG is not well-understood. Here we show that mice with haploinsufficiency of Setd1a, a schizophrenia risk gene encoding a histone H3K4 methyltransferase, develop an enlarged DG with more dentate granule cells after young adulthood. Deletion of Setd1a specifically in quiescent NSCs in the adult DG promotes their activation and neurogenesis, which is countered by inhibition of the histone demethylase LSD1. Mechanistically, RNA-sequencing and CUT & RUN analyses of cultured quiescent adult NSCs reveal Setd1a deletion-induced transcriptional changes and many Setd1a targets, among which down-regulation of Bhlhe40 promotes quiescent NSC activation in the adult DG in vivo. Together, our study reveals a Setd1a-dependent epigenetic mechanism that sustains NSC quiescence in the adult DG.
© 2024. The Author(s).
-
Genetics
-
Neuroscience
-
Stem Cells and Developmental Biology
Sox5 controls the establishment of quiescence in neural stem cells during postnatal development
Preprint on BioRxiv : the Preprint Server for Biology on 3 May 2024 by Medina-Menendez, C., Li, L., et al.
Adult stem cells niches relays in the acquisition of a reversible state of quiescence to ensure long-lasting DNA integrity and cell expansion. Neural stem cells (NSCs) in the dentate gyrus (DG) enter quiescence before the adult hippocampal neurogenic niche is fully established. However, the mechanisms controlling NSC first quiescence entry and quiescence deepness are largely unknown. Using conditional mutant mouse during embryonic or postnatal stages, we have determined that transcription factor Sox5 is required to restrict first entry in quiescence. Moreover, we have found a critical window during the second postnatal week when NSCs build up a shallow or primed quiescent state. Loss of Sox5 leads to an excess of primed NSCs prone to activate leading to a neurogenic burst in the adult DG and precocious depletion of the NSC pool. Mechanistically, Sox5 prevent an excess of BMP canonical signaling activation, a pathway that we have now determined is associated to NSC primed state. In conclusion, our results demonstrate that Sox5 is required to control the correct balance between primed and deep quiescence during the first postnatal weeks of DG development, a balance which is essential for establishing long-lasting adult neurogenesis.
-
Mus musculus (House mouse)
-
Stem Cells and Developmental Biology
Preprint on BioRxiv : the Preprint Server for Biology on 11 April 2024 by Lee, Y., Sarkar, A., et al.
Interleukin-6 (IL-6) is a major pro-inflammatory cytokine for which the levels in plasma demonstrate a robust correlation with age and body mass index (BMI) as part of the senescence-associated secretory phenotype. IL-6 cytokines also play a crucial role in metabolic homeostasis and regenerative processes, primarily via the canonical STAT3 pathway. Thus, selective modulation of IL-6 signaling may offer a unique opportunity for therapeutic interventions. Recently, we discovered that a non-canonical signaling pathway downstream of tyrosine (Y) 814 within the intracellular domain of gp130, the IL-6 co-receptor, is responsible for the recruitment and activation of SRC family of kinases (SFK). Mice with constitutive genetic inactivation of gp130 Y814 (F814 mice) show accelerated resolution of inflammatory response and superior regenerative outcomes in skin wound healing and posttraumatic models of osteoarthritis. The current study was designed to explore if selective genetic or pharmacological inhibition of the non-canonical gp130-Y814/SFK signaling reduces systemic chronic inflammation and multimorbidity in a high-fat diet (HFD)-induced model of accelerated aging. F814 mice showed significantly reduced inflammatory response to HFD in adipose and liver tissue, with significantly reduced levels of systemic inflammation compared to wild type mice. F814 mice were also protected from HFD-induced bone loss and cartilage degeneration. Pharmacological inhibition of gp130-Y814/SFK in mice on HFD mirrored the effects observed in F814 mice on HFD; furthermore, this pharmacological treatment also demonstrated a marked increase in physical activity levels and protective effects against inflammation-associated suppression of neurogenesis in the brain tissue compared to the control group. These findings suggest that selective inhibition of SFK signaling downstream of gp130 receptor represents a promising strategy to alleviate systemic chronic inflammation. Increased degenerative changes and tissue senescence are inevitable in obese and aged organisms, but we demonstrated that the systemic response and inflammation-associated multi-morbidity can be therapeutically mitigated.
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Science Advances on 5 April 2024 by Yan, J., Wu, J., et al.
Despite seizure control by early high-dose pyridoxine (vitamin B6) treatment, at least 75% of pyridoxine-dependent epilepsy (PDE) patients with ALDH7A1 mutation still suffer from intellectual disability. It points to a need for additional therapeutic interventions for PDE beyond pyridoxine treatment, which provokes us to investigate the mechanisms underlying the impairment of brain hemostasis by ALDH7A1 deficiency. In this study, we show that ALDH7A1-deficient mice with seizure control exhibit altered adult hippocampal neurogenesis and impaired cognitive functions. Mechanistically, ALDH7A1 deficiency leads to the accumulation of toxic lysine catabolism intermediates, α-aminoadipic-δ-semialdehyde and its cyclic form, δ-1-piperideine-6-carboxylate, which in turn impair de novo pyrimidine biosynthesis and inhibit NSC proliferation and differentiation. Notably, supplementation of pyrimidines rescues abnormal neurogenesis and cognitive impairment in ALDH7A1-deficient adult mice. Therefore, our findings not only define the important role of ALDH7A1 in the regulation of adult hippocampal neurogenesis but also provide a potential therapeutic intervention to ameliorate the defective mental capacities in PDE patients with seizure control.
-
IHC
-
Mus musculus (House mouse)
-
Genetics
-
Neuroscience
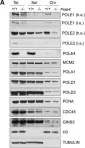
In Mol Cell on 17 May 2018 by Bellelli, R., Borel, V., et al.
Fig.3.A

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Mol Cell by CiteAb, provided under a CC-BY license
Image 1 of 2
In Mol Cell on 17 May 2018 by Bellelli, R., Borel, V., et al.
Fig.4.B

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Mol Cell by CiteAb, provided under a CC-BY license
Image 1 of 2