Activating mutations in p21-activated kinase 1 (PAK1) cause intellectual disability, neurodevelopmental abnormality, macrocephaly, and white matter anomaly in children. Oligodendroglial lineage cells undergo extensive proliferation and population expansion in human and rodent brain during early postnatal development. It remains unclear if and how PAK1 regulates oligodendroglial development. Here, using a series of genetic mouse models, we show that PAK1 controls oligodendroglial progenitor cell (OPC) proliferation and regeneration during normal brain development and in brain white matter injury. Unlike differentiating oligodendrocytes, OPCs display high levels of PAK1 kinase activity which maintains them in a proliferative progenitor state through modulating PDGFRa-mediated mitogenic signaling and acts as a molecular brake limiting OPC differentiation. PAK1-deficient or kinase-inhibited OPCs reduce their proliferation capacity and population expansion in a cell-autonomous manner. Transgenic mice carrying OPC-specific PAK1 deletion or kinase inhibition are populated with fewer OPCs in the homeostatic brain. Furthermore, OPC proliferation and intra-lesional repopulation are significantly impaired in mice of OPC-specific PAK1 deletion or kinase inhibition after white matter injury. Together, our findings suggest that kinase-activating PAK1 mutations stall OPCs in a proliferative progenitor state, impacting timely oligodendroglial differentiation in the CNS of affected children and that PAK1 is a potential molecular target for replenishing OPCs in demyelinating lesions.
© 2025. The Author(s).
Product Citations: 21
In Cellular and Molecular Life Sciences : CMLS on 28 June 2025 by Wang, Y., Kim, B., et al.
-
Biochemistry and Molecular biology
In The Journal of Neuroscience on 17 July 2024 by Etxeberria, A., Shen, Y. A., et al.
Human genetics and preclinical studies have identified key contributions of TREM2 to several neurodegenerative conditions, inspiring efforts to modulate TREM2 therapeutically. Here, we characterize the activities of three TREM2 agonist antibodies in multiple mixed-sex mouse models of Alzheimer's disease (AD) pathology and remyelination. Receptor activation and downstream signaling are explored in vitro, and active dose ranges are determined in vivo based on pharmacodynamic responses from microglia. For mice bearing amyloid-β (Aβ) pathology (PS2APP) or combined Aβ and tau pathology (TauPS2APP), chronic TREM2 agonist antibody treatment had limited impact on microglia engagement with pathology, overall pathology burden, or downstream neuronal damage. For mice with demyelinating injuries triggered acutely with lysolecithin, TREM2 agonist antibodies unexpectedly disrupted injury resolution. Likewise, TREM2 agonist antibodies limited myelin recovery for mice experiencing chronic demyelination from cuprizone. We highlight the contributions of dose timing and frequency across models. These results introduce important considerations for future TREM2-targeting approaches.
Copyright © 2024 the authors.
-
Neuroscience
In The Journal of Clinical Investigation on 15 June 2023 by Sengottuvel, V., Hota, M., et al.
Patients with autosomal recessive microcephaly 15 caused by deficiency in the sodium-dependent lysophosphatidylcholine (LPC) transporter major facilitator superfamily domain-containing 2a (Mfsd2a) present with both microcephaly and hypomyelination, suggesting an important role for LPC uptake by oligodendrocytes in the process of myelination. Here we demonstrate that Mfsd2a is specifically expressed in oligodendrocyte precursor cells (OPCs) and is critical for oligodendrocyte development. Single-cell sequencing of the oligodendrocyte lineage revealed that OPCs from OPC-specific Mfsd2a-KO mice (2aOKO mice) underwent precocious differentiation into immature oligodendrocytes and impaired maturation into myelinating oligodendrocytes, correlating with postnatal brain hypomyelination. 2aOKO mice did not exhibit microcephaly, a finding consistent with the notion that microcephaly is the consequence of an absence of LPC uptake at the blood-brain barrier rather than a deficiency in OPCs. Lipidomic analysis showed that OPCs and iOLs from 2aOKO mice had significantly decreased levels of phospholipids containing omega-3 fatty acids, with a corresponding increase in unsaturated fatty acids, the latter being products of de novo synthesis governed by Srebp-1. RNA-Seq indicated activation of the Srebp-1 pathway and defective expression of regulators of oligodendrocyte development. Taken together, these findings indicate that the transport of LPCs by Mfsd2a in OPCs is important for maintaining OPC state to regulate postnatal brain myelination.
-
Mus musculus (House mouse)
-
Neuroscience
Utilizing mouse optic nerve crush to examine CNS remyelination.
In STAR Protocols on 17 September 2021 by Suter, T. A. C. S., Wang, J., et al.
In developing pro-myelination treatment, an important hurdle is the lack of reliable animal models for assessing de novo myelination in disease settings. We recently showed that regenerated axons in injured optic nerves fail to be myelinated, providing an animal model for this purpose. Here, we describe procedures to promote axonal regeneration, administer optic nerve crush, and assess oligodendrocyte differentiation and maturation into myelination-competent oligodendrocytes. This protocol allows for testing the efficacy of remyelination treatments in an in vivo central nervous system (CNS). For complete details on the use and execution of this protocol, please refer to Wang et al. (2020) and Bei et al. (2016).
© 2021 The Author(s).
-
Neuroscience
In Journal of Histochemistry and Cytochemistry on 1 September 2021 by Ikenari, T., Kawaguchi, T., et al.
Fluoro-Jade C (FJC) staining has been used to detect degenerating neurons in tissue sections. It is a simple and easy staining procedure and does not depend on the manner of cell death. In some experiments, double staining with FJC and fluorescent immunostaining (FI) is required to identify cell types. However, pretreatment for FJC staining contains some processes that are harsh to fluorophores, and the FI signal is greatly reduced. To overcome this issue, we improved the double staining protocol to acquire clear double-stained images by introducing the labeled streptavidin-biotin system. In addition, several studies indicate that FJC can label non-degenerating glial cells, including resting/reactive astrocytes and activated microglia. Moreover, our previous study indicated that degenerating mesenchymal cells were also labeled by FJC, but it is still unclear whether FJC can label degenerating glial cells. Acute encephalopathy model mice contained damaged astrocytes with clasmatodendrosis, and 6-aminonicotinamide-injected mice contained necrotic astrocytes and oligodendrocytes. Using our improved double staining protocol with FJC and FI, we detected FJC-labeled degenerating astrocytes and oligodendrocytes with pyknotic nuclei. These results indicate that FJC is not specific to degenerating neurons in some experimental conditions.
-
Mus musculus (House mouse)
-
Neuroscience
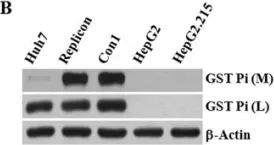
In Sci Rep on 30 June 2017 by Cheng, J. C., Tseng, C. P., et al.
Fig.3.B

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 1