A hallmark of nervous system aging is a decline of white matter volume and function, but the underlying mechanisms leading to white matter pathology are unknown. In the present study, we found age-related alterations of oligodendrocyte cell state with a reduction in total oligodendrocyte density in aging murine white matter. Using single-cell RNA-sequencing, we identified interferon (IFN)-responsive oligodendrocytes, which localize in proximity to CD8+ T cells in aging white matter. Absence of functional lymphocytes decreased the number of IFN-responsive oligodendrocytes and rescued oligodendrocyte loss, whereas T-cell checkpoint inhibition worsened the aging response. In addition, we identified a subpopulation of lymphocyte-dependent, IFN-responsive microglia in the vicinity of the CD8+ T cells in aging white matter. In summary, we provide evidence that CD8+ T-cell-induced, IFN-responsive oligodendrocytes and microglia are important modifiers of white matter aging.
© 2022. The Author(s).
Product Citations: 17
CD8+ T cells induce interferon-responsive oligodendrocytes and microglia in white matter aging.
In Nature Neuroscience on 1 November 2022 by Kaya, T., Mattugini, N., et al.
-
IHC
-
Immunology and Microbiology
-
Neuroscience
T cells induce interferon-responsive oligodendrocytes during white matter aging
Preprint on BioRxiv : the Preprint Server for Biology on 27 March 2022 by Kaya, T., Mattugini, N., et al.
h4>SUMMARY/h4> A hallmark of nervous system aging is a decline of white matter volume and function, but the underlying mechanisms leading to white matter pathology are unknown. Here, we found age-related alterations of oligodendrocytes with a reduction of total oligodendrocyte density in the aging murine white matter. Using single-cell RNA sequencing, we identify interferon-responsive oligodendrocytes, which localize in proximity of CD8 + T cells in the aging white matter. Absence of functional lymphocytes decreased oligodendrocyte reactivity and rescued oligodendrocyte loss, while T-cell checkpoint inhibition worsened the aging affect. In summary, we provide evidence that T cells induced interferon-responsive oligodendrocytes are important modifiers of white matter aging.
-
IHC
-
Mus musculus (House mouse)
-
Immunology and Microbiology
-
Neuroscience
In The Journal of Biological Chemistry on 1 November 2021 by Hyun, S. W., Imamura, A., et al.
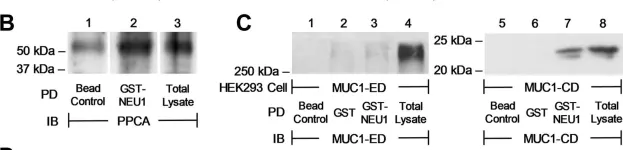
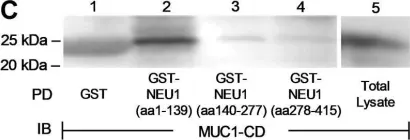
The extracellular domain (ED) of the membrane-spanning sialoglycoprotein, mucin-1 (MUC1), is an in vivo substrate for the lysosomal sialidase, neuraminidase-1 (NEU1). Engagement of the MUC1-ED by its cognate ligand, Pseudomonas aeruginosa-expressed flagellin, increases NEU1-MUC1 association and NEU1-mediated MUC1-ED desialylation to unmask cryptic binding sites for its ligand. However, the mechanism(s) through which intracellular NEU1 might physically interact with its surface-expressed MUC1-ED substrate are unclear. Using reciprocal coimmunoprecipitation and in vitro binding assays in a human airway epithelial cell system, we show here that NEU1 associates with the MUC1-cytoplasmic domain (CD) but not with the MUC1-ED. Prior pharmacologic inhibition of the NEU1 catalytic activity using the NEU1-selective sialidase inhibitor, C9-butyl amide-2-deoxy-2,3-dehydro-N-acetylneuraminic acid, did not diminish NEU1-MUC1-CD association. In addition, glutathione-S-transferase (GST) pull-down assays using the deletion mutants of the MUC1-CD mapped the NEU1-binding site to the membrane-proximal 36 aa of the MUC1-CD. In a cell-free system, we found that the purified NEU1 interacted with the immobilized GST-MUC1-CD and the purified MUC1-CD associated with the immobilized 6XHis-NEU1, indicating that the NEU1-MUC1-CD interaction was direct and independent of its chaperone protein, protective protein/cathepsin A. However, the NEU1-MUC1-CD interaction was not required for the NEU1-mediated MUC1-ED desialylation. Finally, we demonstrated that overexpression of either WT NEU1 or a catalytically dead NEU1 G68V mutant diminished the association of the established MUC1-CD binding partner, PI3K, to MUC1-CD and reduced downstream Akt kinase phosphorylation. These results indicate that NEU1 associates with the juxtamembranous region of the MUC1-CD to inhibit PI3K-Akt signaling independent of NEU1 catalytic activity.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
-
WB
-
Homo sapiens (Human)
-
Biochemistry and Molecular biology
-
Cell Biology
Plasticity-Enhancing Effects of Levodopa Treatment after Stroke.
In International Journal of Molecular Sciences on 23 September 2021 by Talhada, D., Marklund, N., et al.
Dopaminergic treatment in combination with rehabilitative training enhances long-term recovery after stroke. However, the underlying mechanisms on structural plasticity are unknown. Here, we show an increased dopaminergic innervation of the ischemic territory during the first week after stroke induced in Wistar rats subjected to transient occlusion of the middle cerebral artery (tMCAO) for 120 min. This response was also found in rats subjected to permanent focal ischemia induced by photothrombosis (PT) and mice subjected to PT or tMCAO. Dopaminergic branches were detected in the infarct core of mice and rats in both stroke models. In addition, the Nogo A pathway was significantly downregulated in rats treated with levodopa (LD) compared to vehicle-treated animals subjected to tMCAO. Specifically, the number of Nogo A positive oligodendrocytes as well as the levels of Nogo A and the Nogo A receptor were significantly downregulated in the peri-infarct area of LD-treated animals, while the number of Oligodendrocyte transcription factor 2 positive cells increased in this region after treatment. In addition, we observed lower protein levels of Growth Associated Protein 43 in the peri-infarct area compared to sham-operated animals without treatment effect. The results provide the first evidence of the plasticity-promoting actions of dopaminergic treatment following stroke.
-
Cardiovascular biology
In Nutrients on 18 February 2020 by Liu, Y. T., Chen, H. W., et al.
14-Deoxy-11,12-didehydroandrographolide (deAND), a diterpenoid in Andrographis paniculata (Burm. f.) Nees, acts as a bioactive phytonutrient that can treat many diseases. To investigate the protective effects of deAND on reducing fatty liver disease, male mice were fed a high-fat and high-cholesterol (HFHC) diet without or with 0.05% and 0.1% deAND supplementation. Cholesterol accumulation, antioxidant, and anti-inflammatory activities in liver and liver injury were evaluated after deAND treatment. The results show that deAND treatment for seven weeks reduced plasma alanine aminotransferase activity and lowered hepatic cholesterol accumulation, tumor nuclear factor-α, and histological lesions. The 0.1% deAND treatment reduced HFHC diet-induced apoptosis by lowering the caspase 3/pro-caspase 3 ratio. After 11 weeks of deAND treatment, increased NOD-like receptor protein 3 (NLRP3), capase-1, and interleukin-1β protein levels in liver were suppressed by deAND treatment. In addition, nuclear factor erythroid 2-related factor 2 (Nrf2) mRNA expression, heme oxygenase-1 protein expression, and the activities of glutathione peroxidase and glutathione reductase were increased in mice fed the HFHC diet. However, those activities of antioxidant enzymes or proteins were also upregulated by 0.1% deAND treatment. Furthermore, deAND treatment tended to lower hepatic lipid peroxides. Finally, deAND treatment reversed the depletion of hepatic glutamate level induced by the HFHC diet. These results indicate that deAND may ameliorate HFHC diet-induced steatohepatitis and liver injury by increasing antioxidant and anti-inflammatory activities.
-
WB
-
Mus musculus (House mouse)
In J Biol Chem on 1 November 2021 by Hyun, S. W., Imamura, A., et al.
Fig.2.B

-
WB
-
Homo sapiens (Human)
Collected and cropped from J Biol Chem by CiteAb, provided under a CC-BY license
Image 1 of 2
In J Biol Chem on 1 November 2021 by Hyun, S. W., Imamura, A., et al.
Fig.4.C

-
WB
-
Homo sapiens (Human)
Collected and cropped from J Biol Chem by CiteAb, provided under a CC-BY license
Image 1 of 2