Integrin α5β1 is crucial for cell attachment and migration in development and tissue regeneration, and α5β1 binding proteins could have considerable utility in regenerative medicine and next-generation therapeutics. We use computational protein design to create de novo α5β1-specific modulating miniprotein binders, called NeoNectins, that bind to and stabilize the open state of α5β1. When immobilized onto titanium surfaces and throughout 3D hydrogels, the NeoNectins outperform native fibronectin and RGD peptide in enhancing cell attachment and spreading, and NeoNectin-coated titanium implants outperformed fibronectin and RGD-coated implants in animal models in promoting tissue integration and bone growth. NeoNectins should be broadly applicable for tissue engineering and biomedicine. One-Sentence Summary A de novo-designed fibronectin substitute, NeoNectin, is specific for integrin α5β1 and can be incorporated into biomaterials for regenerative medicine.
Product Citations: 34
De Novo Design of Integrin α5β1 Modulating Proteins for Regenerative Medicine
Preprint on BioRxiv : the Preprint Server for Biology on 25 June 2024 by Wang, X., Guillem-Marti, J., et al.
N-cadherin dynamically regulates pediatric glioma cell migration in complex environments.
In The Journal of Cell Biology on 3 June 2024 by Kim, D., Olson, J. M., et al.
Pediatric high-grade gliomas are highly invasive and essentially incurable. Glioma cells migrate between neurons and glia, along axon tracts, and through extracellular matrix surrounding blood vessels and underlying the pia. Mechanisms that allow adaptation to such complex environments are poorly understood. N-cadherin is highly expressed in pediatric gliomas and associated with shorter survival. We found that intercellular homotypic N-cadherin interactions differentially regulate glioma migration according to the microenvironment, stimulating migration on cultured neurons or astrocytes but inhibiting invasion into reconstituted or astrocyte-deposited extracellular matrix. N-cadherin localizes to filamentous connections between migrating leader cells but to epithelial-like junctions between followers. Leader cells have more surface and recycling N-cadherin, increased YAP1/TAZ signaling, and increased proliferation relative to followers. YAP1/TAZ signaling is dynamically regulated as leaders and followers change position, leading to altered N-cadherin levels and organization. Together, the results suggest that pediatric glioma cells adapt to different microenvironments by regulating N-cadherin dynamics and cell-cell contacts.
© 2024 Kim et al.
-
IF
-
Cancer Research
-
Cell Biology
In Autophagy on 1 January 2024 by Kapadia, A., Theil, S., et al.
AD: Alzheimer disease; APP: amyloid beta precursor protein; ATG: autophagy related; Aβ: amyloid-β; CTSD: cathepsin D; DAPI: 4',6-diamidino-2-phenylindole; EEA1: early endosome antigen 1; FA: formic acid; GFP: green fluorescent protein; LAMP2: lysosomal-associated membrane protein 2; MAP1LC3/LC3: microtubule-associated protein 1 light chain 3; MAP2: microtubule-associated protein 2; nmAβ: non-modified amyloid-β; npAβ: non-phosphorylated amyloid-β; pAβ: phosphorylated amyloid-β; p-Ser26Aβ: amyloid-β phosphorylated at serine residue 26; p-Ser8Aβ: amyloid-β phosphorylated at serine residue 8; RAB: RAB, member RAS oncogene family; RFP: red fluorescent protein; SQSTM1/p62: sequestome 1; YFP: yellow fluorescent protein.
-
Cell Biology
In European Journal of Cell Biology on 1 September 2023 by Naslavsky, N. & Caplan, S.
Despite their significance in receptor-mediated internalization and continued signal transduction in cells, early/sorting endosomes (EE/SE) remain incompletely characterized, with many outstanding questions that surround the dynamics of their size and number. While several studies have reported increases in EE/SE size and number resulting from endocytic events, few studies have addressed such dynamics in a methodological and quantitative manner. Herein we apply quantitative fluorescence microscopy to measure the size and number of EE/SE upon internalization of two different ligands: transferrin and epidermal growth factor. Additionally, we used siRNA knock-down to determine the involvement of 5 different endosomal RAB proteins (RAB4, RAB5, RAB8A, RAB10 and RAB11A) in EE/SE dynamics. Our study provides new information on the dynamics of endosomes during endocytosis, an important reference for researchers studying receptor-mediated internalization and endocytic events.
Copyright © 2023 The Authors. Published by Elsevier GmbH.. All rights reserved.
-
Cell Biology
SLC12A9 is a lysosome-detoxifying ammonium – chloride co-transporter
Preprint on BioRxiv : the Preprint Server for Biology on 22 May 2023 by Levin-Konigsberg, R., Mitra, K., et al.
Ammonia is a ubiquitous, toxic by-product of cell metabolism. Its high membrane permeability and proton affinity causes ammonia to accumulate inside acidic lysosomes in its poorly membrane-permeant form: ammonium (NH 4 + ). Ammonium buildup compromises lysosomal function, suggesting the existence of mechanisms that protect cells from ammonium toxicity. Here, we identified SLC12A9 as a lysosomal ammonium exporter that preserves lysosomal homeostasis. SLC12A9 knockout cells showed grossly enlarged lysosomes and elevated ammonium content. These phenotypes were reversed upon removal of the metabolic source of ammonium or dissipation of the lysosomal pH gradient. Lysosomal chloride increased in SLC12A9 knockout cells and chloride binding by SLC12A9 was required for ammonium transport. Our data indicate that SLC12A9 is a chloride-driven ammonium co-transporter that is central in an unappreciated, fundamental mechanism of lysosomal physiology that may have special relevance in tissues with elevated ammonia, such as tumors.
-
Cell Biology
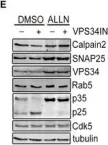
In EMBO J on 2 May 2022 by Liu, G. T., Kochlamazashvili, G., et al.
Fig.5.E

-
WB
-
Collected and cropped from EMBO J by CiteAb, provided under a CC-BY license
Image 1 of 2
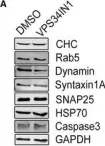
In EMBO J on 2 May 2022 by Liu, G. T., Kochlamazashvili, G., et al.
Fig.3.A

-
WB
-
Collected and cropped from EMBO J by CiteAb, provided under a CC-BY license
Image 1 of 2