L1 cell adhesion molecule (L1CAM)-positive extracellular vesicles (EVs) are being explored as a potential source of biomarkers for Parkinson's disease (PD) in peripheral blood. However, their utility remains controversial. In this study, we sought to investigate the proteome composition of L1CAM+-EVs isolated from human blood plasma and evaluate their potential as biomarkers for PD. L1CAM+-EVs were extracted from blood plasma using direct immunoprecipitation by employing magnetic beads coupled to an anti-L1CAM antibody. The Proximity Extension Assay platform, Olink Explore 3072, was used to analyze samples from 60 individuals: 20 healthy controls (HC), 20 patients with isolated REM sleep behavior disorder (iRBD), and 20 PD patients. Targeted proteomic analysis identified 2841 proteins in L1CAM+-EVs, of which 203 exhibited differential expression across groups. Although these changes were not statistically significant, after correction for multiple testing, a combination of 12 proteins could discriminate between PD and HC. Moreover, several proteins displayed trends toward upregulation or downregulation in PD and iRBD when compared with HC. These preliminary findings suggest that L1CAM+-EVs proteins show some potential as biomarkers for PD; however, further investigation and validation studies are required.
Product Citations: 206
In International Journal of Molecular Sciences on 28 November 2025 by Xylaki, M., Chopra, A., et al.
-
Neuroscience
In International Journal of Molecular Sciences on 8 November 2025 by Latina, V., Soligo, M., et al.
Multi-Wall Carbon Nanotubes (MWCNTs) are under investigation for their use in biomedical applications, especially in neurological diseases, due to their electrochemical properties. Nevertheless, conflicting results have cast doubt on their safety. To advance their translational potential, we evaluated the cytotoxicity of two MWCNT samples in vivo in both vertebrate and invertebrate animal models. Pristine MWCNTs were, in part, used as prepared (MWCNTs), and, in part, annealed at 2400 °C (a-MWCNTs). The two samples differ in their electrochemical properties: MWCNTs are not electro-conductive, while a-MWCNTs are electro-conductive and negatively charged on their surface. We evaluated the effects of both intranasally delivered MWCNTs on several key markers of cell viability in the olfactory bulbs and hippocampus from healthy adult Wistar rats, as well as their impact on lifespan, genotoxicity, oxidative stress, and aging-related functional markers in the nematode Caenorhabditis elegans. Neither of the two MWCNT samples was cytotoxic towards neuronal cells in the hippocampus. In olfactory bulbs, only electro-conductive a-MWCNTs interacted with two positively charged mitochondrial proteins: Translocase of Outer Mitochondrial Membrane 20 (TOM20) and Cytochrome C (CytC). In C. elegans, neither type of MWCNT affected lifespan or brood size, and cytosolic ROS levels remained unchanged. Notably, treated worms exhibited a significantly delayed aging phenotype. Metallic MWCNTs are biocompatible in living organisms and possess the potential to modulate neural cells functioning in vivo.
α-Synuclein purification significantly impacts seed amplification assay performance and consistency.
In Acta Neuropathologica Communications on 5 November 2025 by Al-Azzawi, Z. A. M., Silver, N. R. G., et al.
α-Synuclein seed amplification assays are a promising diagnostic tool for synucleinopathies such as Parkinson's disease and multiple system atrophy. Standardized conditions are required to ensure a high degree of inter- and intra-laboratory reproducibility when performing these assays. A significant issue that hinders the utility of seed amplification assays is the de novo aggregation propensity of the α-synuclein substrate as well as inter-batch heterogeneity. While much work has focused on determining appropriate seed amplification assay buffer compositions as well as the type and amount of seed used, a robust comparison of α-synuclein substrate purification methods has not been reported. We therefore compared the utility of recombinant α-synuclein purified using four different methods as seed amplification assay substrates across two laboratories. Osmotic shock-purified α-synuclein monomer substrate showed the lowest propensity for de novo aggregation, which translated into being the best substrate for seed amplification assay reactions seeded with α-synuclein preformed fibrils or patient brain homogenates. Furthermore, osmotic shock α-synuclein monomer showed the best inter-batch reproducibility compared to all other substrates tested. As α-synuclein seed amplification assays continue to evolve and move towards adoption in the clinical realm, this work showcases the vital importance of standardizing the production and characterization of recombinant α-synuclein substrate. We encourage the widespread adoption of osmotic shock α-synuclein monomer as the universal substrate for seed amplification assays to maximize intra- and inter-laboratory reproducibility.
© 2025. The Author(s).
-
WB
-
Plant Science
In Molecular Neurodegeneration on 31 October 2025 by Morella, M. L., Al Khayrat, B., et al.
The abnormal accumulation of alpha-Synuclein (αSyn) within neurons is a hallmark of synucleinopathies, such as Parkinson's disease (PD), and could stem from impaired protein degradation. Genetic, in vitro, and post-mortem studies have suggested that lysosomal dysfunction and impaired proteolytic activity play important roles in the pathogenesis of PD. Lysosomes have been proposed as key sites for αSyn degradation, but direct evidence of the lysosomal localization of endogenous αSyn in the human brain is limited. This study aimed to investigate the localization of αSyn proteoforms, including different post-translational modifications (PTMs), within lysosomes of post-mortem human nigral neurons. We analyzed formalin-fixed, paraffin-embedded brain tissue from donors diagnosed with PD, PD with Dementia (PDD) or incidental Lewy body disease (iLBD). Substantia nigra sections were assessed using an extensive panel of αSyn-specific antibodies, including PTM-specific antibodies, and selected lysosomal markers via multiplex immunofluorescence, confocal and stimulated emission depletion (STED) microscopy. Here, we demonstrate widespread accumulation of αSyn within lysosomes in nigral dopaminergic neuron somas of donors with PD/PDD and iLBD. This lysosomal αSyn appeared morphologically distinct from cytosolic inclusions such as Lewy bodies (LBs) and related macro-aggregates, and was present both in cells with and without these larger αSyn deposits. When present, macro-aggregates were consistently accompanied by ring-shaped lysosomal structures. Compared to other neuronal morphologies, lysosomal αSyn was the most frequent morphology at early Braak stages (1-4), with a decline at later stages (5-6). Interestingly, lysosomal αSyn was detected solely by targeting the N-terminus or the NAC domain of αSyn, and not with antibodies targeting Serine 129-phosphorylated αSyn or other epitopes at the C-terminus (CT), suggesting that lysosome-associated αSyn lacks the CT. Our findings reveal two co-existing pools of neuronal somatic αSyn: a CT-negative lysosome-associated form, and a primarily non-lysosomal CT-positive form. Overall, we provide direct evidence of lysosomal involvement in cellular αSyn metabolism in post-mortem human PD brain.
© 2025. The Author(s).
-
Cell Biology
-
Neuroscience
Targeted α-Synuclein mRNA Degradation by PMO-Based RNA-Degrading Chimeras
Preprint on BioRxiv : the Preprint Server for Biology on 18 October 2025 by Wang, N., Hegde, S., et al.
α-Synucleinopathies are devastating neurodegenerative diseases characterized by pathological accumulation of a neuronal protein, α-synuclein (αSyn). Lowering soluble αSyn levels is a promising therapeutic strategy to limit aggregation and neurotoxicity, but directly targeting this protein is hindered by its intrinsically disordered structure and other factors, such as its conformational heterogeneity and intracellular drug delivery barriers. Consequently, increasing attention has been directed toward targeting the SNCA transcript, which encodes αSyn. Here, we developed phosphorodiamidate morpholino oligonucleotide (PMO)-based RNA-degrading chimeras (RDCs) that selectively bind the 5′ untranslated region of SNCA mRNA and recruit RNase L for targeted RNA degradation. Through the systematic evaluation of 10 RDCs, we identified and optimized 4-D1, which effectively reduced SNCA mRNA and αSyn protein expression in HEK293T cells in an RNase L-dependent manner. 4-D1 lowered SNCA transcript and αSyn protein levels in both primary cortical neurons from humanized SNCA mice and in human induced pluripotent stem cell-derived cortical neurons. This reduction prevented prion-like seeding induced by patient-derived αSyn fibrils and protected neurons from fibril-induced cytotoxicity. Finally, in vivo studies confirmed the efficacy of 4-D1 in reducing αSyn mRNA expression in humanized SNCA mice. These findings indicate that PMO-based RDCs may represent a promising therapeutic modality for α-synucleinopathies. Significance Statement Abnormal aggregation of the neuronal protein α-synuclein is central to Parkinson’s disease and related disorders, yet therapeutic candidates that directly target this protein have yet to demonstrate efficacy. in clinical trials. We developed a new strategy that lowers α-synuclein production at the RNA level using phosphorodiamidate morpholino oligonucleotide (PMO)–based RNA-degrading chimeras (RDCs). These molecules recruit a natural RNA-degrading enzyme to selectively destroy the RNA transcript encoding α-synuclein. Our lead RDC reduced α-synuclein levels in cultured cells, humanized mouse and human neurons, blocked α-synuclein pathological aggregation, and protected neurons from toxicity. This study establishes RDCs as a promising therapeutic platform for Parkinson’s disease and other neurodegenerative diseases driven by α-synuclein.
-
WB
-
Genetics
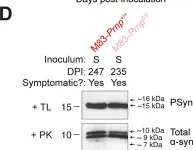
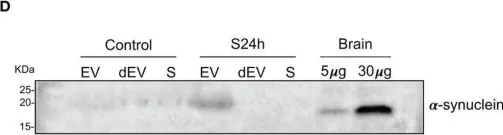
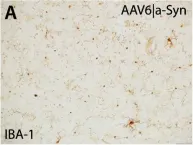
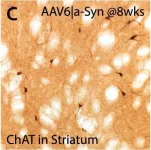
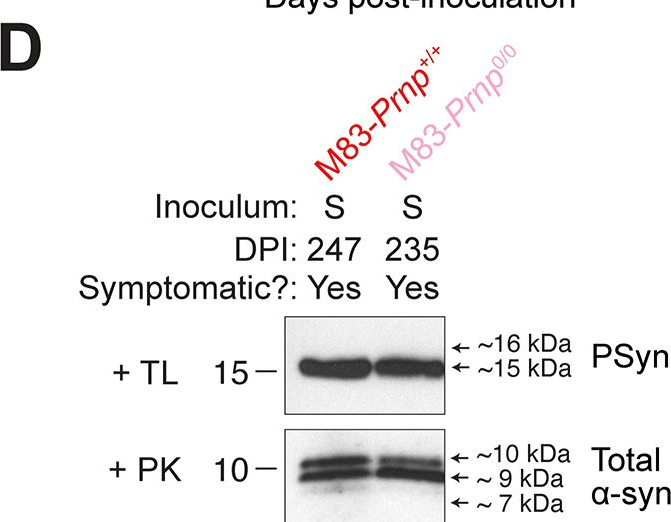
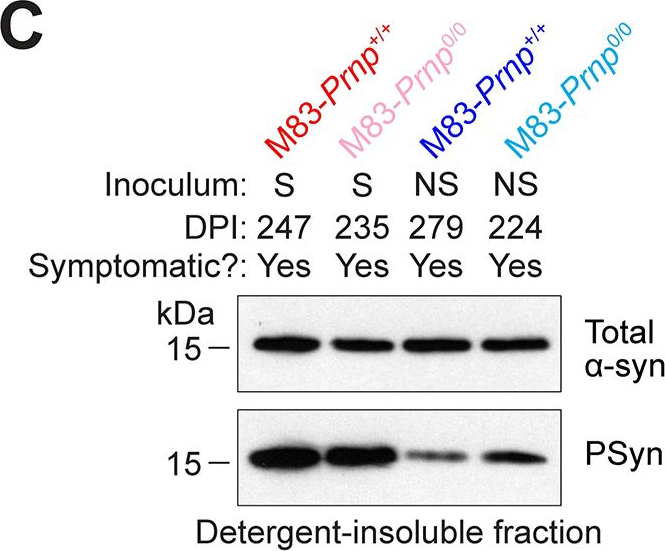
In PLoS Pathog on 1 September 2024 by So, R. W. L., Amano, G., et al.
Fig.6.D
-
WB
-
Collected and cropped from PLoS Pathogens by CiteAb, provided under a CC-BY license
Image 1 of 26
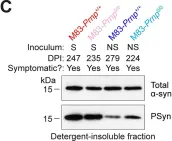
In PLoS Pathog on 1 September 2024 by So, R. W. L., Amano, G., et al.
Fig.6.C
-
WB
-
Collected and cropped from PLoS Pathogens by CiteAb, provided under a CC-BY license
Image 1 of 26
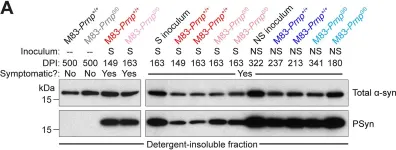
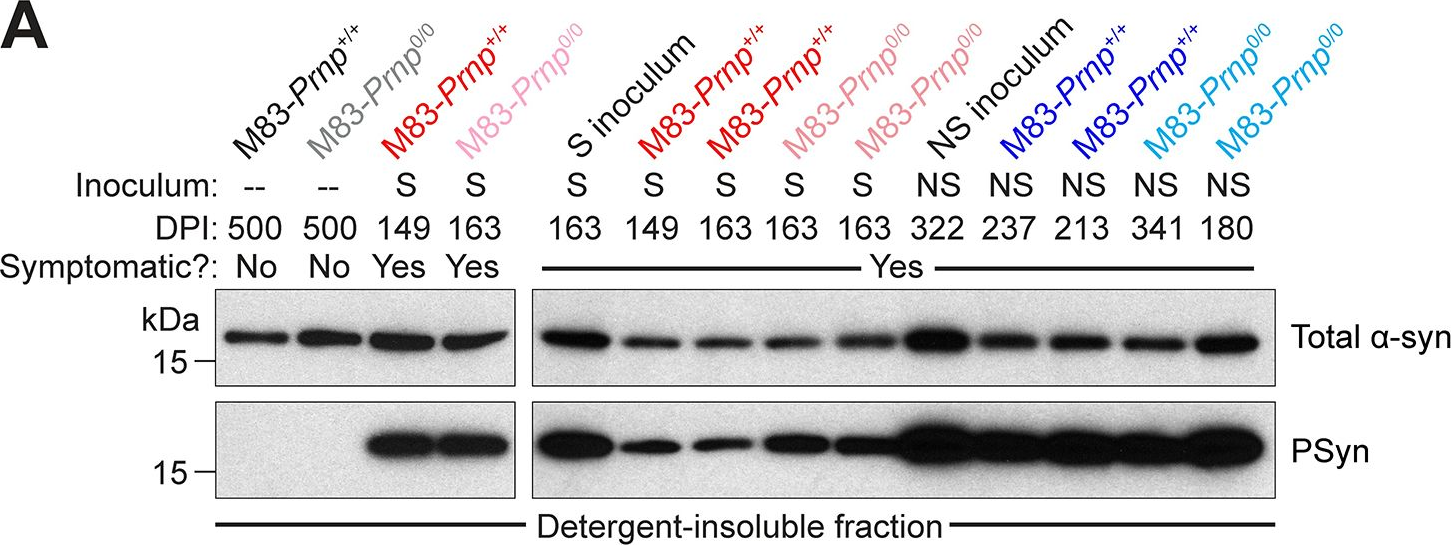
In PLoS Pathog on 1 September 2024 by So, R. W. L., Amano, G., et al.
Fig.2.A
-
WB
-
Collected and cropped from PLoS Pathogens by CiteAb, provided under a CC-BY license
Image 1 of 26
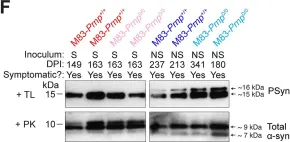
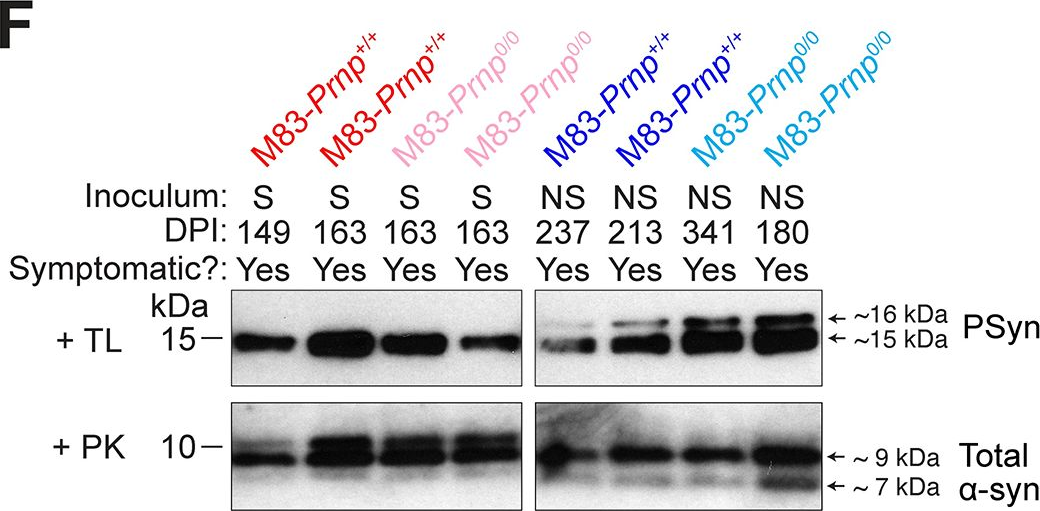
In PLoS Pathog on 1 September 2024 by So, R. W. L., Amano, G., et al.
Fig.2.F
-
WB
-
Collected and cropped from PLoS Pathogens by CiteAb, provided under a CC-BY license
Image 1 of 26
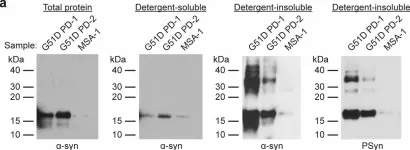
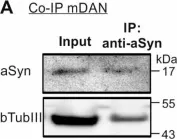
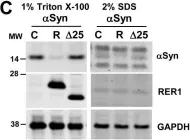
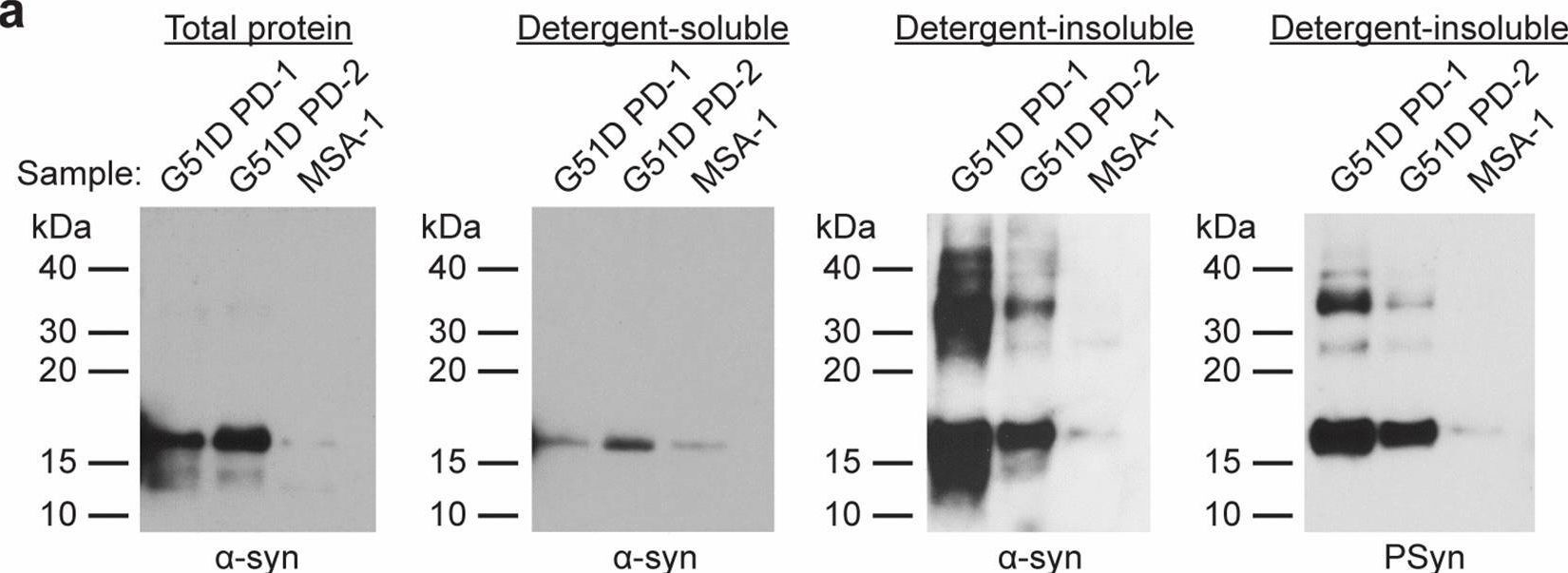
In Acta Neuropathol Commun on 3 May 2023 by Lau, H. H. C., Martínez-Valbuena, I., et al.
Fig.1.A
-
WB
-
Collected and cropped from Acta Neuropathologica Communications by CiteAb, provided under a CC-BY license
Image 1 of 26
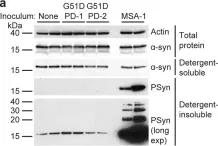
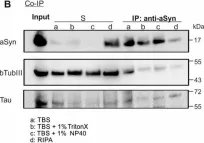
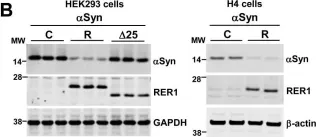
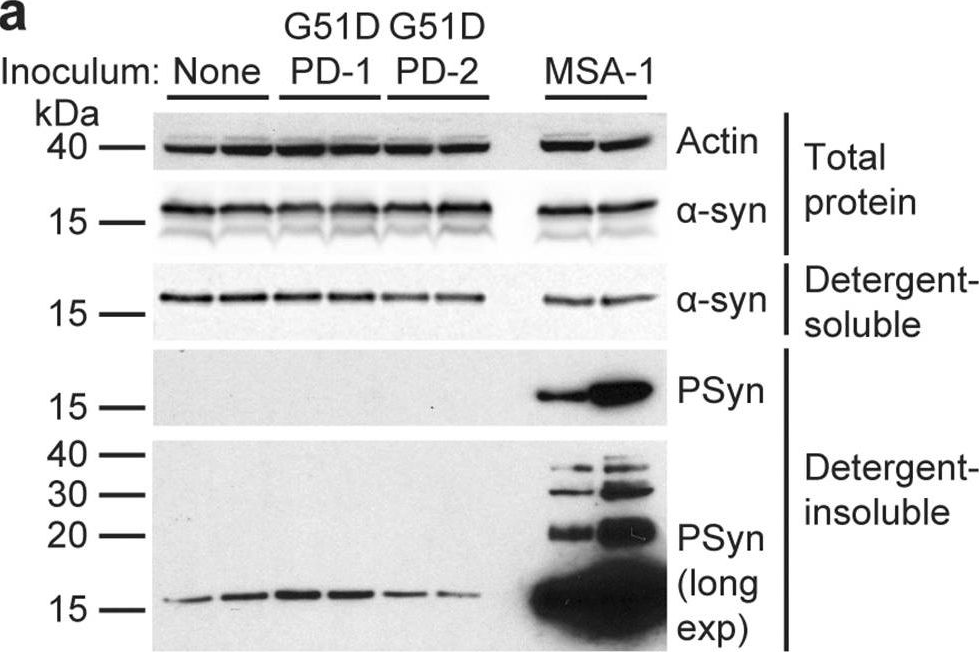
In Acta Neuropathol Commun on 3 May 2023 by Lau, H. H. C., Martínez-Valbuena, I., et al.
Fig.2.A
-
WB
-
Collected and cropped from Acta Neuropathologica Communications by CiteAb, provided under a CC-BY license
Image 1 of 26
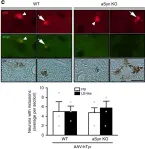
In Int J Mol Sci on 5 February 2022 by Seebauer, L., Schneider, Y., et al.
Fig.5.B
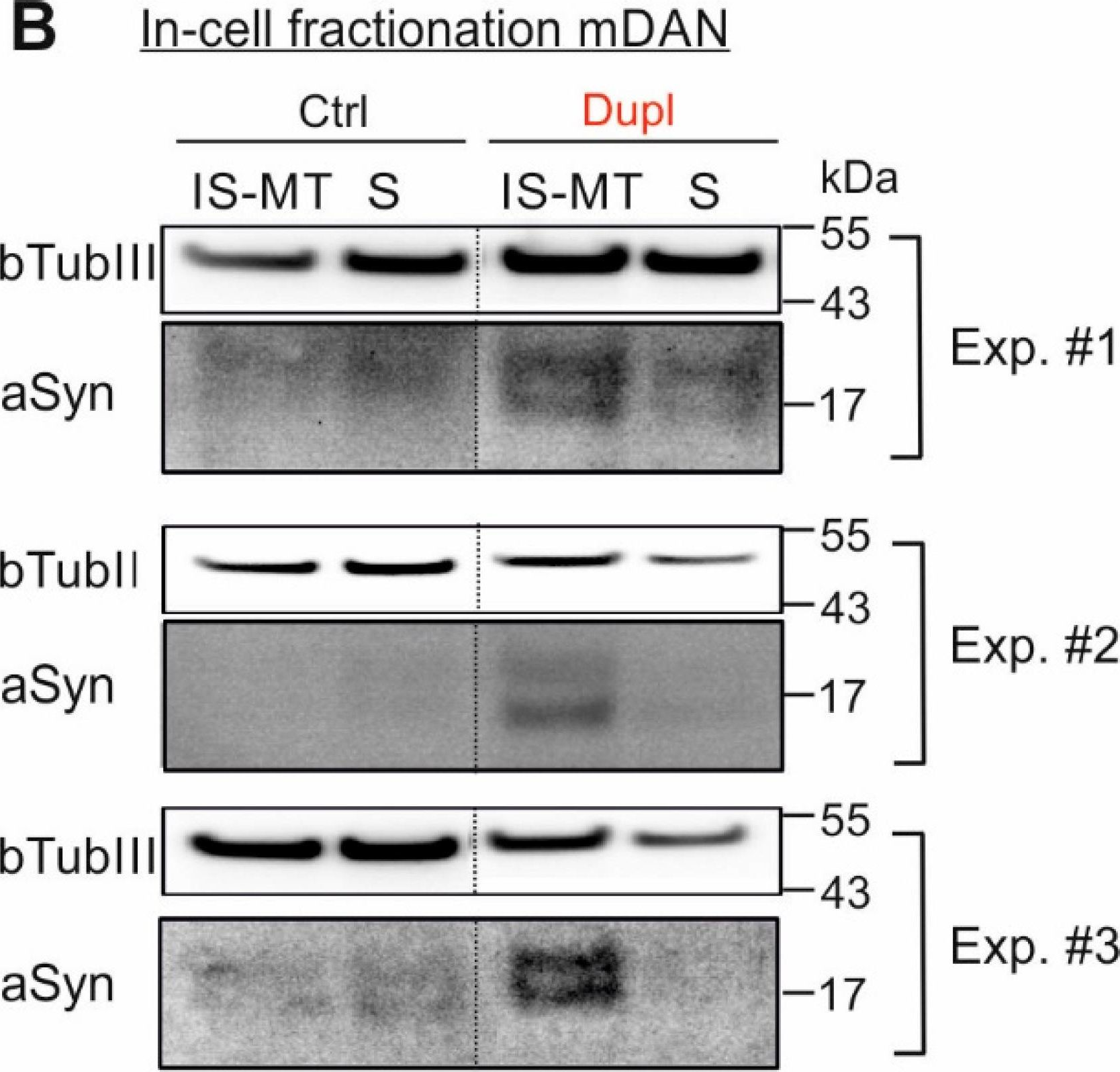
-
WB
-
Homo sapiens (Human)
Collected and cropped from International Journal of Molecular Sciences by CiteAb, provided under a CC-BY license
Image 1 of 26
In Int J Mol Sci on 5 February 2022 by Seebauer, L., Schneider, Y., et al.
Fig.2.B
-
WB
-
Homo sapiens (Human)
Collected and cropped from International Journal of Molecular Sciences by CiteAb, provided under a CC-BY license
Image 1 of 26
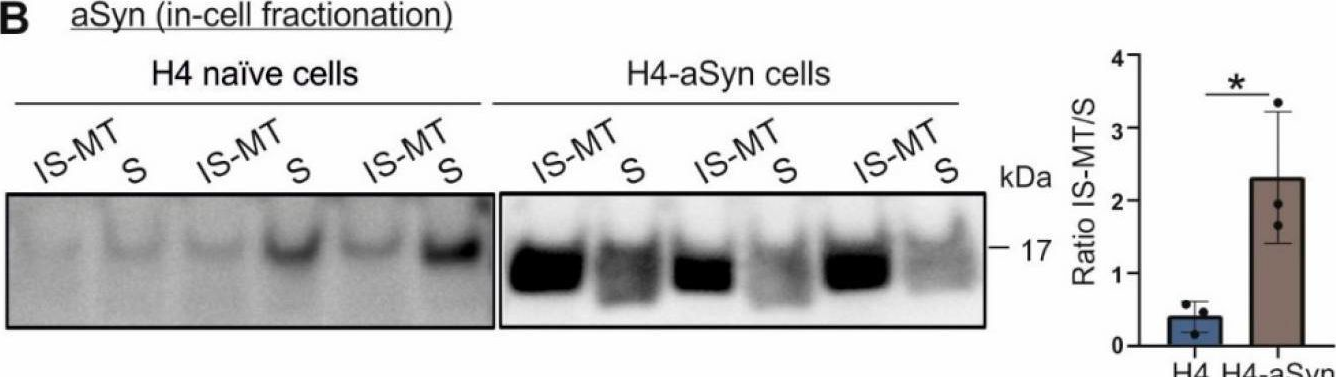
In Front Immunol on 5 February 2022 by Mkrtchian, S., Ebberyd, A., et al.
Fig.4.D
-
WB
-
Collected and cropped from Frontiers in Immunology by CiteAb, provided under a CC-BY license
Image 1 of 26
In Int J Mol Sci on 5 February 2022 by Seebauer, L., Schneider, Y., et al.
Fig.5.A
-
WB
-
Homo sapiens (Human)
Collected and cropped from International Journal of Molecular Sciences by CiteAb, provided under a CC-BY license
Image 1 of 26
In Int J Mol Sci on 5 February 2022 by Seebauer, L., Schneider, Y., et al.
Fig.1.B
-
WB
-
Homo sapiens (Human)
Collected and cropped from International Journal of Molecular Sciences by CiteAb, provided under a CC-BY license
Image 1 of 26
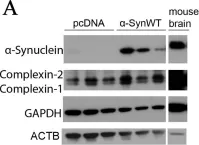
In Acta Neuropathol Commun on 9 March 2021 by Latina, V., Giacovazzo, G., et al.
Fig.4.A
-
WB
-
Mus musculus (House mouse)
Collected and cropped from Acta Neuropathologica Communications by CiteAb, provided under a CC-BY license
Image 1 of 26
In Nat Commun on 7 March 2019 by Carballo-Carbajal, I., Laguna, A., et al.
Fig.7.C
-
IHC-P-IF
-
Rattus norvegicus (Rat)
Collected and cropped from Nature Communications by CiteAb, provided under a CC-BY license
Image 1 of 26
In Aging Cell on 1 August 2018 by Stankova, T., Piepkorn, L., et al.
Fig.4.B
-
WB
-
Mus musculus (House mouse)
Collected and cropped from Aging Cell by CiteAb, provided under a CC-BY license
Image 1 of 26
In PLoS One on 7 September 2017 by Park, H. J., Ryu, D., et al.
Fig.4.D
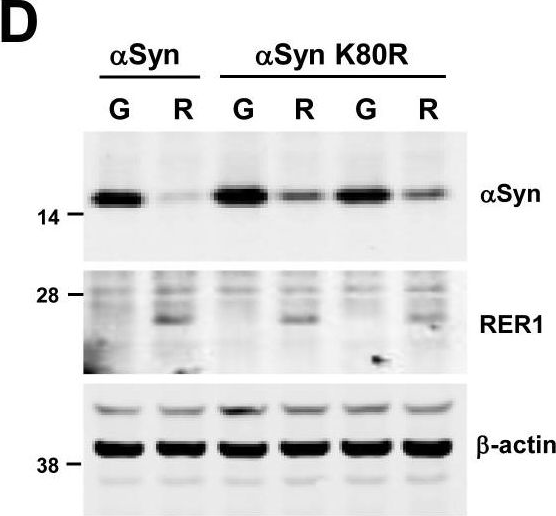
-
WB
-
Homo sapiens (Human)
Collected and cropped from PLoS ONE by CiteAb, provided under a CC-BY license
Image 1 of 26
In PLoS One on 7 September 2017 by Park, H. J., Ryu, D., et al.
Fig.4.C
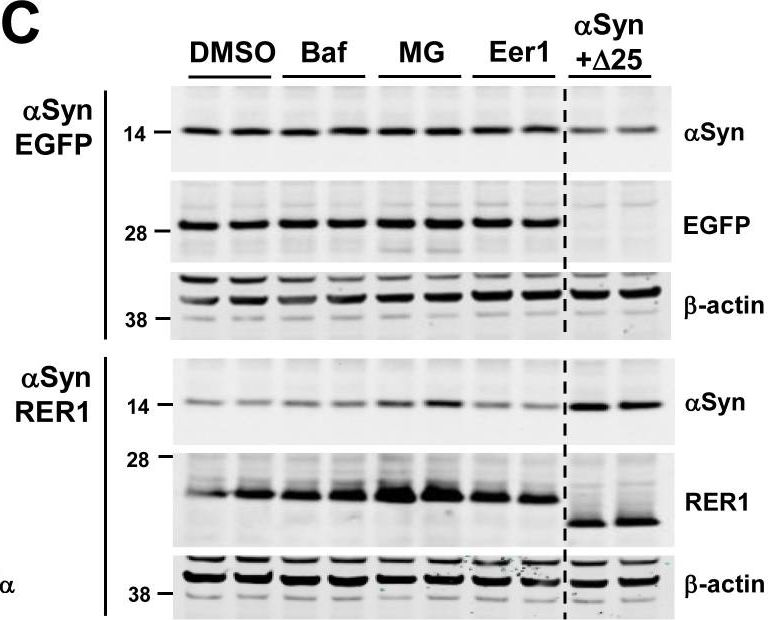
-
WB
-
Homo sapiens (Human)
Collected and cropped from PLoS ONE by CiteAb, provided under a CC-BY license
Image 1 of 26
In PLoS One on 7 September 2017 by Park, H. J., Ryu, D., et al.
Fig.4.A
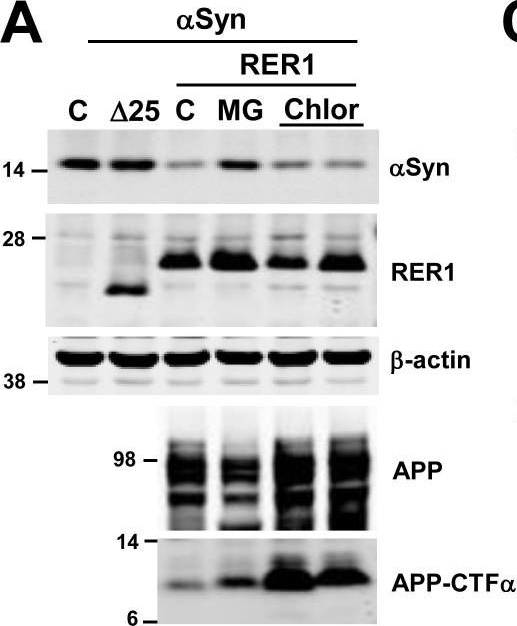
-
WB
-
Homo sapiens (Human)
Collected and cropped from PLoS ONE by CiteAb, provided under a CC-BY license
Image 1 of 26
In PLoS One on 7 September 2017 by Park, H. J., Ryu, D., et al.
Fig.2.C
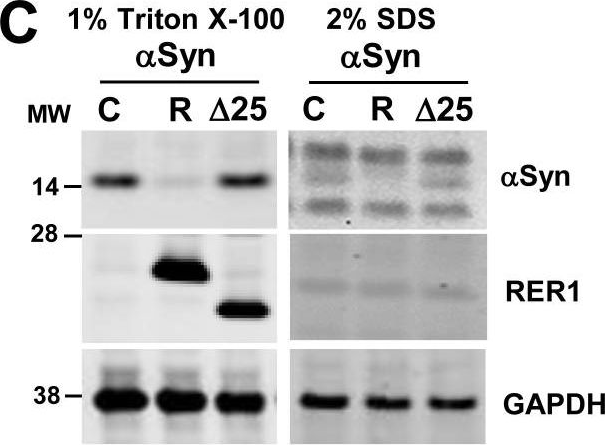
-
WB
-
Homo sapiens (Human)
Collected and cropped from PLoS ONE by CiteAb, provided under a CC-BY license
Image 1 of 26
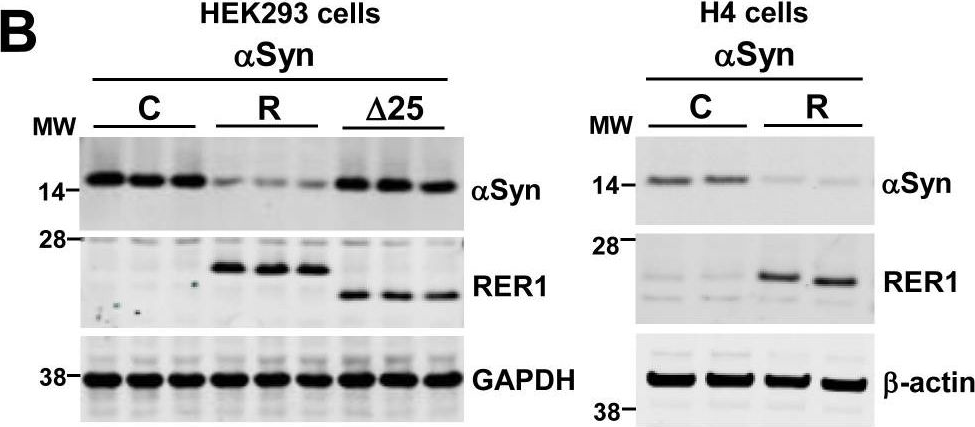
In PLoS One on 7 September 2017 by Park, H. J., Ryu, D., et al.
Fig.2.B
-
WB
-
Homo sapiens (Human)
Collected and cropped from PLoS ONE by CiteAb, provided under a CC-BY license
Image 1 of 26
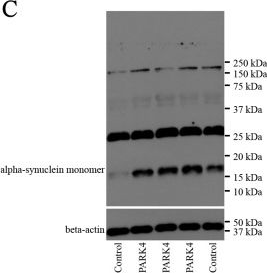
In Dis Model Mech on 1 May 2017 by Lahut, S., Gispert, S., et al.
Fig.1.C
-
WB
-
Mus musculus (House mouse)
Collected and cropped from Disease Models & Mechanisms by CiteAb, provided under a CC-BY license
Image 1 of 26
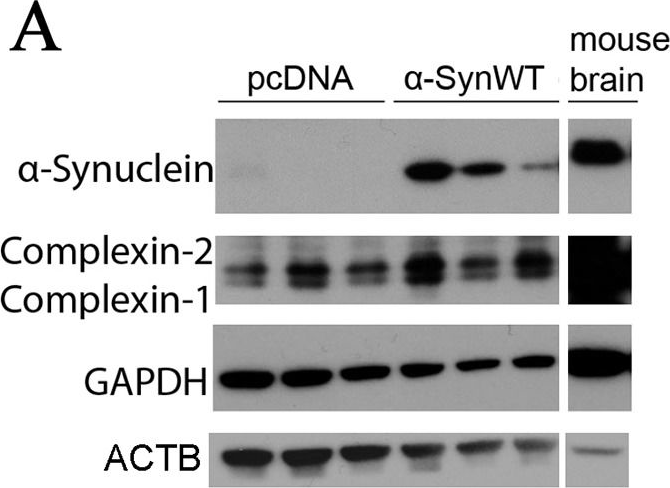
In Dis Model Mech on 1 May 2017 by Lahut, S., Gispert, S., et al.
Fig.5.A
-
WB
-
Homo sapiens (Human)
Collected and cropped from Disease Models & Mechanisms by CiteAb, provided under a CC-BY license
Image 1 of 26
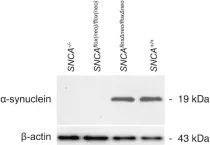
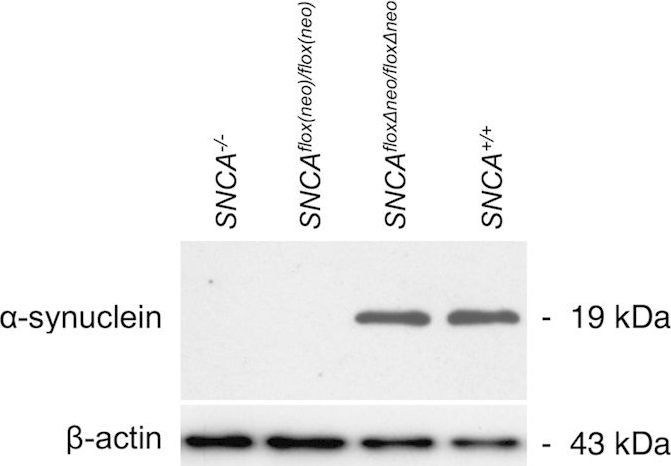
In Sci Rep on 13 November 2015 by Ninkina, N., Connor-Robson, N., et al.
Fig.2.A
-
WB
-
Mus musculus (House mouse)
Collected and cropped from Scientific Reports by CiteAb, provided under a CC-BY license
Image 1 of 26
In PLoS One on 8 July 2014 by Aldrin-Kirk, P., Davidsson, M., et al.
Fig.10.A
-
IHC-IF
-
Rattus norvegicus (Rat)
Collected and cropped from PLoS ONE by CiteAb, provided under a CC-BY license
Image 1 of 26
In PLoS One on 8 July 2014 by Aldrin-Kirk, P., Davidsson, M., et al.
Fig.9.C
-
WB
-
Rattus norvegicus (Rat)
Collected and cropped from PLoS ONE by CiteAb, provided under a CC-BY license
Image 1 of 26
In PLoS One on 17 November 2011 by Maarouf, C. L., Daugs, I. D., et al.
Fig.7.A
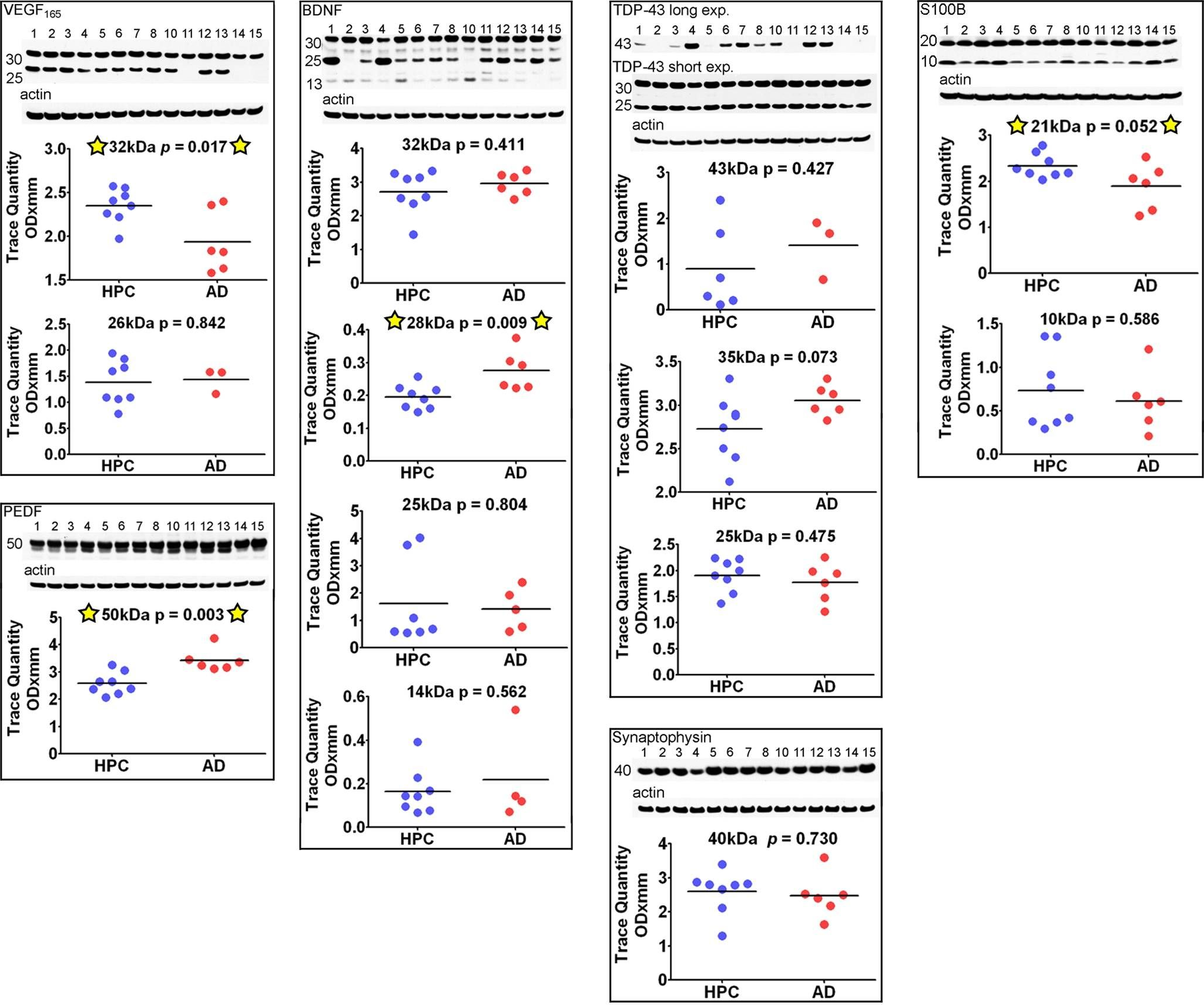
-
WB
-
Homo sapiens (Human)
Collected and cropped from PLoS ONE by CiteAb, provided under a CC-BY license
Image 1 of 26