Previous research indicates that carcinogenesis involves disrupting the functions of numerous genes, including factors involved in the regulation of transcription and cell proliferation. For these reasons, in endometrial carcinogenesis, we decided to investigate the expression of TSG101 (a suppressor of tumor transformation) and LSF (a transcription factor involved in numerous cellular processes, such as cell cycle regulation, cell growth, development, and apoptosis). LSF may be involved in the regulation of TSG101 expression. The research material consisted of endometrial cancer samples from 60 patients. The control group consisted of normal endometrium samples donated by 60 women undergoing surgery for benign diseases of the female reproductive organs. The samples were subjected to immunohistochemical staining with antibodies specific to TSG101 and LSF. Specific antibodies were used to identify TSG101 and LSF in the examined histopathological preparations. An approximately 14-fold lower risk of endometrial cancer development was observed in patients with TSG expression in more than 75% of the assessed cells (4% vs. 36%; OR = 0.07; p = 0.0182). There was a four-fold lower risk of endometrial cancer development in patients with LSF expression in more than 50% of the assessed cells (32% vs. 64%; OR = 0.26; p = 0.0262). A more than three-fold lower risk of endometrial cancer development was observed in patients with LSF expression in more than 75% of the assessed cells (24% vs. 52%; OR = 0.29; p = 0.0454). Endometrial cancer was diagnosed in those with a lower level of TSG101 expression than in those with a cancer-free endometrium. Decreased expression of TSG101 may be a marker of endometrial cancer, and increased expression of LSF when diagnosed with endometrial cancer may indicate greater advancement of the disease. These markers might be used as diagnostic and prognostic markers-however, there is a lack of a correlation between them.
Product Citations: 12
In Cells on 26 March 2024 by Ziemiński, R., Stupak, A., et al.
-
IHC
-
Homo sapiens (Human)
-
Biochemistry and Molecular biology
-
Cell Biology
Prognostic Implication of YY1 and CP2c Expression in Patients with Primary Breast Cancer.
In Cancers on 4 July 2023 by Cha, C. D., Son, S. H., et al.
Yin Yang 1 (YY1) is a transcription factor that regulates epigenetic pathways and protein modifications. CP2c is a transcription factor that functions as an oncogene to regulate cell proliferation. YY1 is known to interact with CP2c to suppress CP2c's transcriptional activity. This study aimed to investigate YY1 and CP2c expression in breast cancer and prognostic implications. In this study, YY1 and CP2c expression was evaluated using immunohistochemical staining, Western blot and RT-PCR assays. Of 491 patients with primary breast cancer, 138 patients showed YY1 overexpression. Luminal subtype and early stage were associated with overexpression (p < 0.001). After a median follow-up of 68 months, YY1 overexpression was found to be associated with a better prognosis (disease-free survival rates of 92.0% vs. 79.2%, p = 0.014). In Cox proportional hazards model, YY1 overexpression functioned as an independent prognostic factor after adjustment of hormone receptor/HER2 status and tumor size (hazard ratio of 0.50, 95% CI 0.26-0.98, p = 0.042). Quantitative analysis of YY1 and CP2c protein expression in tumors revealed a negative correlation between them. In conclusion, YY1 overexpression is a favorable prognostic biomarker in patients with breast cancer, and it has a negative correlation with CP2c at the protein level.
-
IHC
-
Cancer Research
In The American Journal of Pathology on 1 August 2022 by Lotfollahzadeh, S., Lo, D., et al.
Aberrant hyperactivation of Wnt signaling, driven by nuclear β-catenin in the colonic epithelium, represents the seminal event in the initiation and progression of colorectal cancer (CRC). Despite its established role in CRC tumorigenesis, clinical translation of Wnt inhibitors remains unsuccessful. Late SV40 factor (LSF; encoded by TFCP2) is a transcription factor and a potent oncogene. The current study identified a chemotype, named factor quinolinone inhibitors (FQIs), that specifically inhibits LSF DNA-binding, partner protein-binding, and transactivation activities. The role of LSF and FQIs in CRC tumor growth was examined. Herein, the study showed that LSF and β-catenin interacted in several CRC cell lines irrespective of their mutational profile, which was disrupted by FQI2-34. FQI2-34 suppressed Wnt activity in CRC cells in a dose-dependent manner. Leveraging both allogeneic and syngeneic xenograft models showed that FQI2-34 suppressed CRC tumor growth, significantly reduced nuclear β-catenin, and down-regulated Wnt targets such as axis inhibition protein 2 (AXIN-2) and SRY-box transcription factor 9, in the xenograft cells. FQI2-34 suppressed the proliferation of xenograft cells. Adenocarcinomas from a series of stage IV CRC patients revealed a positive correlation between LSF expression and Wnt targets (AXIN-2 and SRY-box transcription factor 9) within the CRC cells. Collectively, this study uncovers the Wnt inhibitory and CRC growth-suppressive effects of these LSF inhibitors in CRC cells, revealing a novel target in CRC therapeutics.
Copyright © 2022 American Society for Investigative Pathology. All rights reserved.
-
Cancer Research
-
Pathology
In Ginekologia Polska on 25 January 2022 by Stupak, A., Kwaśniewski, W., et al.
The presence of the endometrium outside the uterine cavity affects about 10% of women of childbearing age. Studies of the progression of endometriosis to cancer have been supported by numerous evidences of gene expression or gene defect caused by oxidative stress and inflammation. We decided to check the expression of selected factors responsible for the proliferation, as in the stages of neoplasia.
A group of 80 women with ovary localization of endometriosis was qualified for research. The control group was 90 patients with ovarian simplex or follicular cysts. The DNA isolation, immunohistochemical analysis of IGF 1, IGF-R, TSG 101, and LSF expressions with a quantitative scoring of slides and electron microscopy was performed.
The IGF-1-immunopositive cells in the reference group were in statistically significantly higher number compared to the cells forming the foci of endometriosis (p = 0.0282). However, the number of IGF-R-immunopositive cells was comparable to the endometriosis (p = 0.1264). In the control group, the number of LSF-immunopositive cells was statistically significantly higher in comparison to endometriosis foci (p = 0.000001), but the number of TSG 101-immunositive cells was comparable to endometriosis foci (p = 0.3834). A weak negative correlation between the number of cells expressing the TSG 101 factor and the IGF-1 receptor was found in the endometriosis group (r = -0.26, p = 0.0196). The analysis of CA single nucleotide polymorphism in the DNA isolated from both groups showed a comparable incidence of MSS and MSI-L genotypes (chi2 p = 0,9160).
How these factors affect the development of endometriosis and whether they could be helpful in the diagnosis requires further research.
-
Homo sapiens (Human)
Nogo-B promotes invasion and metastasis of nasopharyngeal carcinoma via RhoA-SRF-MRTFA pathway.
In Cell Death & Disease on 24 January 2022 by Wang, J., Zhong, Q., et al.
Distant metastasis remains the major cause for treatment failure in patients with nasopharyngeal carcinoma (NPC). Thus, it is necessary to investigate the underlying regulation mechanisms and potential biomarkers for NPC metastasis. Nogo-B (neurite outgrowth inhibitor B), encoded by reticulon-4, has been shown to be associated with the progression and advanced stage of several cancer types. However, the relationship between Nogo-B and NPC remains unknown. In this study, we found that higher expression of Nogo-B was detected in NPC cells and tissues. Higher expression of Nogo-B was statistically relevant to N stage, M stage, and poor prognosis in NPC patients. Further functional investigations indicated that Nogo-B overexpression could increase the migration, invasion, and metastasis ability of NPC cells in vitro and in vivo. Mechanistically, Nogo-B promoted epithelial-mesenchymal transition (EMT) and enhanced the invasive potency by interacting directly with its receptor NgR3 in NPC. Additionally, overexpression of Nogo-B could upregulate the protein levels of p-RhoA, SRF, and MRTFA. A positive relationship was found between the expression of Nogo-B and the p-RhoA in NPC patients as well as in mouse lung xenografts. Nogo-Bhigh p-RhoAhigh expression was significantly associated with N stage, M stage, and poor prognosis in NPC patients. Notably, CCG-1423, an inhibitor of the RhoA-SRF-MRTFA pathway, could reverse the invasive potency of Nogo-B and NgR3 in NPC cell lines, and decrease the expression of N-Cadherin, indicating that CCG-1423 may be a potential target drug of NPC. Taken together, our findings reveal that Nogo-B enhances the migration and invasion potency of NPC cells via EMT by binding to its receptor NgR3 to regulate the RhoA-SRF-MRTFA pathway. These findings could provide a novel insight into understanding the metastasis mechanism and targeted therapy of advanced NPC.
© 2022. The Author(s).
-
IHC
-
Homo sapiens (Human)
-
Cancer Research
-
Cell Biology
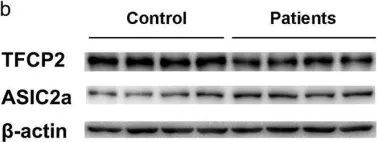
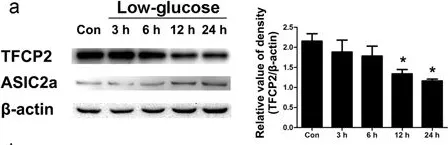
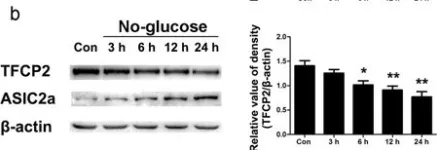
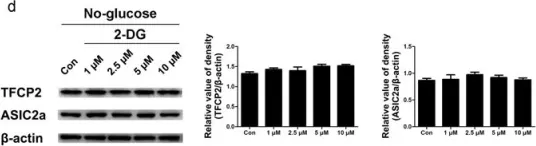
In Sci Rep on 19 July 2017 by Zhang, H., Gao, G., et al.
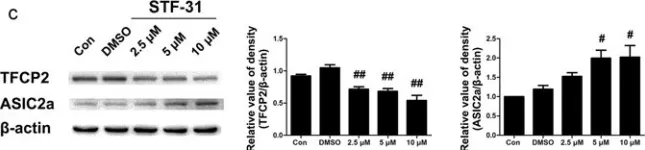
Fig.1.B

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 9
In Sci Rep on 19 July 2017 by Zhang, H., Gao, G., et al.
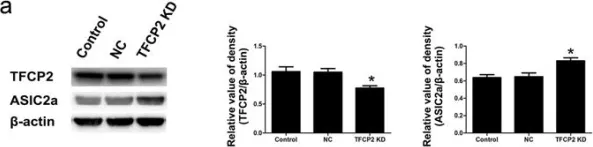
Fig.2.C

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 9
In Sci Rep on 19 July 2017 by Zhang, H., Gao, G., et al.
Fig.3.A

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 9
In Sci Rep on 19 July 2017 by Zhang, H., Gao, G., et al.
Fig.3.B

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 9
In Sci Rep on 19 July 2017 by Zhang, H., Gao, G., et al.
Fig.3.D

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 9
In Sci Rep on 19 July 2017 by Zhang, H., Gao, G., et al.
Fig.3.C

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 9
In Sci Rep on 19 July 2017 by Zhang, H., Gao, G., et al.
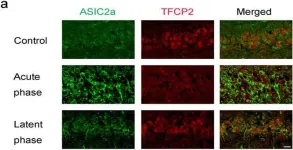
Fig.4.A

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 9
In Sci Rep on 19 July 2017 by Zhang, H., Gao, G., et al.
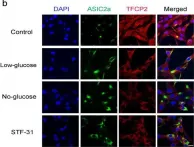
Fig.5.A

-
IHC-IF
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 9
In Sci Rep on 19 July 2017 by Zhang, H., Gao, G., et al.
Fig.5.B

-
ICC-IF
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 9