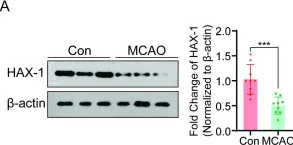
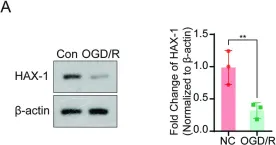
Acute cerebral ischemia has a high rate of disability and death. Although timely recanalization therapy may rescue the ischemic brain tissue, cerebral ischemia-reperfusion injury has been shown to limit the therapeutic effects of vascular recanalization. Protein HAX-1 has been reported as a pro-survival protein that plays an important role in various disorders, particularly in association with the nervous system. However, the effects and mechanisms of HAX-1 in cerebral IR injury have yet to be elucidated. So, we aimed to investigate the effect of HAX-1 on microglial pyroptosis and explore its potential neuroprotective effects in ischemia-reperfusion injury. Our results show that the expression of HAX-1 decreased after cerebral IR injury, accompanied by an increase in pyroptosis pathway activation. In addition, HAX-1 could inhibit microglial pyroptosis both in vivo and in vitro and reduce the release of inflammatory mediators. The above neuroprotective effects might be partially mediated by inhibiting of interaction of NLRP3 and ASC through competitive binding, followed by the attenuation of NLRP3 inflammasome formation. In conclusion, Our findings support that HAX-1 exhibits a protective role in cerebral I/R injury, and further study on HAX-1 expression regulation will contribute to cerebral infarction therapy.
© 2024. The Author(s).
Product Citations: 5
HAX-1 interferes in assembly of NLRP3-ASC to block microglial pyroptosis in cerebral I/R injury.
In Cell Death Discovery on 29 May 2024 by Guo, X. B., Deng, X., et al.
-
WB
-
Mus musculus (House mouse)
-
Neuroscience
In Cellular Microbiology on 1 September 2021 by Chatterjee, S., Lekmeechai, S., et al.
Many enteric pathogens employ a type III secretion system (T3SS) to translocate effector proteins directly into the host cell cytoplasm, where they subvert signalling pathways of the intestinal epithelium. Here, we report that the anti-apoptotic regulator HS1-associated protein X1 (HAX-1) is an interaction partner of the T3SS effectors EspO of enterohaemorrhagic Escherichia coli (EHEC) and Citrobacter rodentium, OspE of Shigella flexneri and Osp1STYM of Salmonella enterica serovar Typhimurium. EspO, OspE and Osp1STYM have previously been reported to interact with the focal adhesions protein integrin linked kinase (ILK). We found that EspO localizes both to the focal adhesions (ILK localisation) and mitochondria (HAX-1 localisation), and that increased expression of HAX-1 leads to enhanced mitochondrial localisation of EspO. Ectopic expression of EspO, OspE and Osp1STYM protects cells from apoptosis induced by staurosporine and tunicamycin. Depleting cells of HAX-1 indicates that the anti-apoptotic activity of EspO is HAX-1 dependent. Both HAX-1 and ILK were further confirmed as EspO1-interacting proteins during infection using T3SS-delivered EspO1. Using cell detachment as a proxy for cell death we confirmed that T3SS-delivered EspO1 could inhibit cell death induced during EPEC infection, to a similar extent as the anti-apoptotic effector NleH, or treatment with the pan caspase inhibitor z-VAD. In contrast, in cells lacking HAX-1, EspO1 was no longer able to protect against cell detachment, while NleH1 and z-VAD maintained their protective activity. Therefore, during both infection and ectopic expression EspO protects cells from cell death by interacting with HAX-1. These results suggest that despite the differences between EHEC, C. rodentium, Shigella and S. typhimurium infections, hijacking HAX-1 anti-apoptotic signalling is a common strategy to maintain the viability of infected cells. TAKE AWAY: EspO homologues are found in EHEC, Shigella, S. typhimurium and some EPEC. EspO homologues interact with HAX-1. EspO protects infected cells from apoptosis. EspO joins a growing list of T3SS effectors that manipulate cell death pathways.
© 2021 The Authors. Cellular Microbiology published by John Wiley & Sons Ltd.
-
WB
-
Immunology and Microbiology
In Journal of Molecular Recognition : JMR on 1 July 2016 by Qian, L., Bradford, A. M., et al.
Growth factor receptor bound protein 7 (Grb7) is a signal-transducing adaptor protein that mediates specific protein-protein interactions in multiple signaling pathways. Grb7, with Grb10 and Grb14, is members of the Grb7 protein family. The topology of the Grb7 family members contains several protein-binding domains that facilitate the formation of protein complexes, and high signal transduction efficiency. Grb7 has been found overexpressed in several types of cancers and cancer cell lines and is presumed involved in cancer progression through promotion of cell proliferation and migration via interactions with the erythroblastosis oncogene B 2 (human epidermal growth factor receptor 2) receptor, focal adhesion kinase, Ras-GTPases, and other signaling partners. We previously reported Grb7 binds to Hax1 (HS1 associated protein X1) isoform 1, an anti-apoptotic protein also involved in cell proliferation and calcium homeostasis. In this study, we confirm that the in vitro Grb7/Hax1 interaction is exclusive to these two proteins and their interaction does not depend on Grb7 dimerization state. In addition, we report Grb7 and Hax1 isoform 1 may colocalize partially to mitochondria in epidermal growth factor-treated SKBR3 cells and growth conditions can affect this colocalization. Moreover, Grb7 can affect Caspase3 cleavage of Hax1 isoform 1 in vitro, and Grb7 expression may slow Caspase3 cleavage of Hax1 isoform 1 in apoptotic HeLa cells. Finally, Grb7 is shown to increase cell viability in apoptotic HeLa cells in a time-dependent manner. Taken together, these discoveries provide clues for the role of a Grb7/Hax1 protein interaction in apoptosis pathways involving Hax1. Copyright © 2016 John Wiley & Sons, Ltd.
Copyright © 2016 John Wiley & Sons, Ltd.
-
Cell Biology
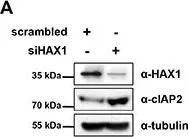
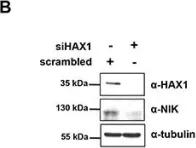
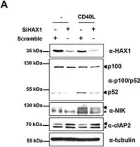
HAX1 regulates E3 ubiquitin ligase activity of cIAPs by promoting their dimerization.
In Oncotarget on 30 October 2014 by Choi, J. S., Park, B. C., et al.
HS-1-associated protein X-1 (HAX1) is a multi-functional protein which was first identified as a Hematopoietic cell specific Lyn Substrate 1 (HS1)-binding protein. Although the roles of HAX1 in apoptosis have been unraveled and HAX1 has been proposed to be involved in several diseases, additional roles of HAX1 are still being identified. Here, we demonstrated that HAX1 directly interacted with cellular Inhibitor of Apoptosis Proteins (cIAPs), ubiquitin E3 ligases which regulate the abundance of cellular proteins, via ubiquitin-dependent proteasomal degradation. We showed that HAX1 promotes auto-ubiquitination and degradation of cIAPs by facilitating the intermolecular homodimerization of RING finger domain. Moreover, HAX1 regulates the non-canonical Nuclear Factor-κB (NF-κB) signaling pathway by modulating the stability of NF-κB-Inducing Kinase (NIK), which is one of the substrates of cIAPs. Taken together, these results unveil a novel role of HAX1 in the non-canonical NF-κB pathway, and provide an important clue that HAX1 is a potential therapeutic target for the treatment of cancer.
-
WB
-
Homo sapiens (Human)
In Journal of Virology on 1 January 2013 by Hsu, W. B., Shih, J. L., et al.
Transcription and replication of the influenza A virus RNA genome occur in the nucleus through the viral RNA-dependent RNA polymerase consisting of PB1, PB2, and PA. Cellular factors that associate with the viral polymerase complex play important roles in these processes. To look for cellular factors that could associate with influenza A virus PA protein, we have carried out a yeast two-hybrid screen using a HeLa cell cDNA library. We identified six cellular proteins that may interact with PA. We focused our study on one of the new PA-interacting proteins, HAX1, a protein with antiapoptotic function. By using glutathione S-transferase pulldown and coimmunoprecipitation assays, we demonstrate that HAX1 specifically interacts with PA in vitro and in vivo and that HAX1 interacts with the nuclear localization signal domain of PA. Nuclear accumulation of PA was increased in HAX1-knockdown cells, and this phenotype could be reversed by reexpression of HAX1, indicating that HAX1 can impede nuclear transport of PA. As a consequence, knockdown of HAX1 resulted in a significant increase in virus yield and polymerase activity in a minigenome assay, and this phenotype could be reversed by reexpression of HAX1, indicating that HAX1 can inhibit influenza A virus propagation. Together, these results not only provide insight into the mechanism underlying nuclear transport of PA but also identify an intrinsic host factor that restricts influenza A virus infection.
-
Immunology and Microbiology
In Cell Death Discov on 29 May 2024 by Guo, X. B., Deng, X., et al.
Fig.2.A

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Cell Death Discov by CiteAb, provided under a CC-BY license
Image 1 of 5
In Cell Death Discov on 29 May 2024 by Guo, X. B., Deng, X., et al.
Fig.3.A

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Cell Death Discov by CiteAb, provided under a CC-BY license
Image 1 of 5
In Oncotarget on 30 October 2014 by Choi, J. S., Park, B. C., et al.
Fig.2.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 5
In Oncotarget on 30 October 2014 by Choi, J. S., Park, B. C., et al.
Fig.5.B

-
WB
-
Homo sapiens (Human)
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 5
In Oncotarget on 30 October 2014 by Choi, J. S., Park, B. C., et al.
Fig.6.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 5