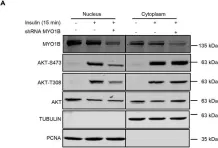
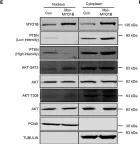
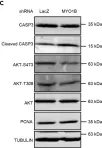
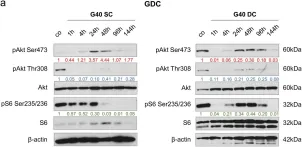
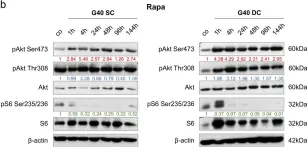
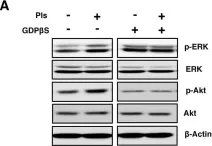
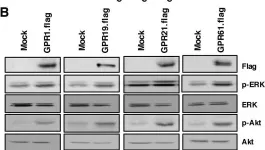
Breast cancer includes tumor subgroups with morphological, molecular, and clinical differences. Intrinsic heterogeneity especially characterizes breast tumors with a triple negative phenotype, often leading to the failure of even the most advanced therapeutic strategies. To improve breast cancer treatment, the use of natural agents to integrate conventional therapies is the subject of ever-increasing attention. In this context, garlic (Allium sativum) shows anti-cancerous potential, interfering with the proliferation, motility, and malignant progression of both non-invasive and invasive breast tumor cells. As heterogeneity could be at the basis of variable effects, the main objective of our study was to evaluate the anti-tumoral activity of a garlic extract in breast cancer cells with a triple negative phenotype. Established triple negative breast cancer (TNBC) cell lines from patient-derived xenografts (PDXs) were used, revealing subtype-dependent effects on morphology, cell cycle, and invasive potential, correlated with the peculiar down-modulation of Akt signaling, a crucial regulator in solid tumors. Our results first demonstrate that the effects of garlic on TNBC breast cancer are not unique and suggest that only more precise knowledge of the mechanisms activated by this natural compound in each tumor will allow for the inclusion of garlic in personalized therapeutic approaches to breast cancer.
Product Citations: 49
In Cells on 11 May 2024 by Brugnoli, F., Dell'Aira, M., et al.
-
Homo sapiens (Human)
-
Cancer Research
-
Cell Biology
IGF1 promotes human myotube differentiation toward a mature metabolic and contractile phenotype.
In American Journal of Physiology - Cell Physiology on 1 May 2024 by Dreher, S. I., Grubba, P., et al.
Skeletal muscle mediates the beneficial effects of exercise, thereby improving insulin sensitivity and reducing the risk for type 2 diabetes. Current human skeletal muscle models in vitro are incapable of fully recapitulating its physiological functions especially muscle contractility. By supplementation of insulin-like growth factor 1 (IGF1), a growth factor secreted by myofibers in vivo, we aimed to overcome these limitations. We monitored the differentiation process starting from primary human CD56-positive myoblasts in the presence/absence of IGF1 in serum-free medium in daily collected samples for 10 days. IGF1-supported differentiation formed thicker multinucleated myotubes showing physiological contraction upon electrical pulse stimulation (EPS) following day 6. Myotubes without IGF1 were almost incapable of contraction. IGF1 treatment shifted the proteome toward skeletal muscle-specific proteins that contribute to myofibril and sarcomere assembly, striated muscle contraction, and ATP production. Elevated PPARGC1A, MYH7, and reduced MYH1/2 suggest a more oxidative phenotype further demonstrated by higher abundance of proteins of the respiratory chain and elevated mitochondrial respiration. IGF1-treatment also upregulated glucose transporter (GLUT)4 and increased insulin-dependent glucose uptake compared with myotubes differentiated without IGF1. To conclude, addition of IGF1 to serum-free medium significantly improves the differentiation of human myotubes that showed enhanced myofibril formation, response to electrical pulse stimulation, oxidative respiratory capacity, and glucose metabolism overcoming limitations of previous standards. This novel protocol enables investigation of muscular exercise on a molecular level.NEW & NOTEWORTHY Human skeletal muscle models are highly valuable to study how exercise prevents type 2 diabetes without invasive biopsies. Current models did not fully recapitulate the function of skeletal muscle especially during exercise. By supplementing insulin-like growth factor 1 (IGF1), the authors developed a functional human skeletal muscle model characterized by inducible contractility and increased oxidative and insulin-sensitive metabolism. The novel protocol overcomes the limitations of previous standards and enables investigation of exercise on a molecular level.
-
WB
-
Biochemistry and Molecular biology
-
Cell Biology
-
Endocrinology and Physiology
In Biomolecules on 31 October 2023 by zur Nedden, S., Safari, M. S., et al.
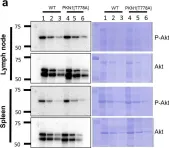
We recently identified protein kinase N1 (PKN1) as a negative gatekeeper of neuronal AKT protein kinase activity during postnatal cerebellar development. The developing cerebellum is specifically vulnerable to hypoxia-ischemia (HI), as it occurs during hypoxic-ischemic encephalopathy, a condition typically caused by oxygen deprivation during or shortly after birth. In that context, activation of the AKT cell survival pathway has emerged as a promising new target for neuroprotective interventions. Here, we investigated the role of PKN1 in an in vitro model of HI, using postnatal cerebellar granule cells (Cgc) derived from Pkn1 wildtype and Pkn1-/- mice. Pkn1-/- Cgc showed significantly higher AKT phosphorylation, resulting in reduced caspase-3 activation and improved survival after HI. Pkn1-/- Cgc also showed enhanced axonal outgrowth on growth-inhibitory glial scar substrates, further pointing towards a protective phenotype of Pkn1 knockout after HI. The specific PKN1 phosphorylation site S374 was functionally relevant for the enhanced axonal outgrowth and AKT interaction. Additionally, PKN1pS374 shows a steep decrease during cerebellar development. In summary, we demonstrate the pathological relevance of the PKN1-AKT interaction in an in vitro HI model and establish the relevant PKN1 phosphorylation sites, contributing important information towards the development of specific PKN1 inhibitors.
-
IP
-
Biochemistry and Molecular biology
-
Neuroscience
Engineering of human myotubes toward a mature metabolic and contractile phenotype
Preprint on BioRxiv : the Preprint Server for Biology on 12 June 2023 by Dreher, S. I., Grubba, P., et al.
Skeletal muscle mediates the beneficial effects of exercise, thereby improving insulin sensitivity and reducing the risk for type 2 diabetes. Current human skeletal-muscle-models in vitro are incapable of fully recapitulating its physiological functions, especially contractility. By supplementation of insulin-like growth factor 1 (IGF1), secreted by myofibers in vivo, we overcome these limitations. We monitored the differentiation process starting from primary human CD56-positive myoblasts in the presence/absence of IGF1 in serum-free medium daily over 10 days. IGF1-supported differentiation formed thicker multinucleated myotubes showing physiological contraction upon electrical-pulse-stimulation following day 6. Myotubes without IGF1 were almost incapable of contraction. IGF1-treatment shifted the proteome toward skeletal muscle-specific proteins that contribute to myofibril and sarcomere assembly, striated muscle contraction, and ATP production. Elevated PPARGC1A, MYH7 and reduced MYH1/2 suggest a more oxidative phenotype further demonstrated by higher abundance of respiratory chain proteins and elevated mitochondrial respiration. IGF1-treatment also upregulated GLUT4 and increased insulin-dependent glucose uptake compared to myotubes differentiated without IGF1. To conclude, utilizing IGF1, we engineered human myotubes that recapitulate the physiological traits of skeletal muscle in vivo vastly superior to established protocols. This novel model enables investigation of exercise on a molecular level, is suitable for drug screening and studies on interorgan-crosstalk. Table of Contents Human skeletal muscle models are highly valuable to study how exercise prevents type 2 diabetes without invasive biopsies. Current models did not fully recapitulate the function of adult muscle especially during exercise. By supplementing insulin-like growth factor 1 (IGF1) the authors engineered a functional human skeletal muscle model characterized by inducible contractility and increased oxidative and insulin-sensitive metabolism. The novel protocol overcomes the limitations of previous standards and enables investigation of exercise on a molecular level, drug screening and interactions between organs in organ-on-chip.
-
Biochemistry and Molecular biology
-
Cell Biology
In International Journal of Molecular Sciences on 30 December 2022 by Sharma, D., Rasool, F., et al.
Cancer is one of the leading cause of lethality worldwide, CRC being the third most common cancer reported worldwide, with 1.85 million cases and 850,000 deaths annually. As in all other cancers, kinases are one of the major enzymes that play an essential role in the incidence and progression of CRC. Thus, using multi-kinase inhibitors is one of the therapeutic strategies used to counter advanced-stage CRC. Regorafenib is an FDA-approved drug in the third-line therapy of refractory metastatic colorectal cancer. Acquired resistance to cancers and higher toxicity of these drugs are disadvantages to the patients. To counter this, combination therapy is used as a strategy where a minimal dose of drugs can be used to get a higher efficacy and reduce drug resistance development. Ruthenium-based compounds are observed to be a potential alternative to platinum-based drugs due to their significant safety and effectiveness. Formerly, our lab reported Ru-1, a ruthenium-based compound, for its anticancer activity against multiple cancer cells, such as HepG2, HCT116, and MCF7. This study evaluates Ru-1's activity against regorafenib-resistant HCT116 cells and as a combination therapeutic with regorafenib. Meanwhile, the mechanism of the effect of Ru-1 alone and with regorafenib as a combination is still unknown. In this study, we tested a drug combination (Ru-1 and regorafenib) against a panel of HT29, HCT116, and regorafenib-resistant HCT116 cells. The combination showed a synergistic inhibitory activity. Several mechanisms underlying these numerous synergistic activities, such as anti-proliferative efficacy, indicated that the combination exhibited potent cytotoxicity and enhanced apoptosis induction. Disruption of mitochondrial membrane potential increased intracellular ROS levels and decreased migratory cell properties were observed. The combination exhibited its activity by regulating PI3K/Akt and p38 MAP kinase signalling. This indicates that the combination of REG/Ru-1 targets cancer cells by modulating the PI3K/Akt and ERK signalling.
-
WB
-
Cancer Research
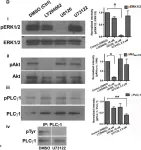
In Sci Rep on 27 September 2019 by Siddique, S. M., Kubouchi, K., et al.
Fig.6.A

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 13
In iScience on 27 September 2019 by Yu, Y., Xiong, Y., et al.
Fig.1.A

-
WB
-
Collected and cropped from iScience by CiteAb, provided under a CC-BY license
Image 1 of 13
In iScience on 27 September 2019 by Yu, Y., Xiong, Y., et al.
Fig.3.E

-
WB
-
Collected and cropped from iScience by CiteAb, provided under a CC-BY license
Image 1 of 13
In iScience on 27 September 2019 by Yu, Y., Xiong, Y., et al.
Fig.6.C

-
WB
-
Collected and cropped from iScience by CiteAb, provided under a CC-BY license
Image 1 of 13
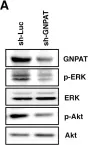
In Oncogenesis on 29 November 2017 by Langhans, J., Schneele, L., et al.
Fig.3.A

-
WB
-
Collected and cropped from Oncogenesis by CiteAb, provided under a CC-BY license
Image 1 of 13
In Oncogenesis on 29 November 2017 by Langhans, J., Schneele, L., et al.
Fig.3.B

-
WB
-
Collected and cropped from Oncogenesis by CiteAb, provided under a CC-BY license
Image 1 of 13
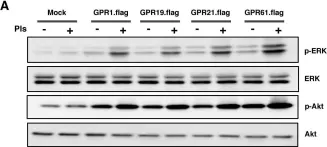
In PLoS One on 5 March 2016 by Hossain, M. S., Mineno, K., et al.
Fig.1.A

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 13
In PLoS One on 5 March 2016 by Hossain, M. S., Mineno, K., et al.
Fig.3.B

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 13
In PLoS One on 5 March 2016 by Hossain, M. S., Mineno, K., et al.
Fig.4.A

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 13
In PLoS One on 5 March 2016 by Hossain, M. S., Mineno, K., et al.
Fig.5.A

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 13
In PLoS One on 5 March 2016 by Hossain, M. S., Mineno, K., et al.
Fig.5.C

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 13
In Front Mol Neurosci on 30 August 2013 by Chan, W. S., Sideris, A., et al.
Fig.3.D

-
WB
-
Rattus norvegicus (Rat)
Collected and cropped from Front Mol Neurosci by CiteAb, provided under a CC-BY license
Image 1 of 13
In BMC Cancer on 25 August 2011 by Wedel, S., Hudak, L., et al.
Fig.8.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from BMC Cancer by CiteAb, provided under a CC-BY license
Image 1 of 13