The epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase with important roles in many cellular processes as well as in cancer and other diseases. EGF binding promotes EGFR dimerization and autophosphorylation through interactions that are well understood structurally. How these dimers relate to higher-order EGFR oligomers seen in cell membranes, however, remains unclear. Here, we used single-particle tracking (SPT) and Förster resonance energy transfer imaging to examine how each domain of EGFR contributes to receptor oligomerization and the rate of receptor diffusion in the cell membrane. Although the extracellular region of EGFR is sufficient to drive receptor dimerization, we find that the EGF-induced EGFR slowdown seen by SPT requires higher-order oligomerization-mediated in part by the intracellular tyrosine kinase domain when it adopts an active conformation. Our data thus provide important insight into the interactions required for higher-order EGFR assemblies involved in EGF signaling.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Product Citations: 35
Distinct interactions stabilize EGFR dimers and higher-order oligomers in cell membranes.
In Cell Reports on 23 January 2024 by Mudumbi, K. C., Burns, E. A., et al.
-
WB
In PLoS Pathogens on 1 December 2023 by So, C. W., Sourisseau, M., et al.
The multi-step process of hepatitis C virus (HCV) entry is facilitated by various host factors, including epidermal growth factor receptor (EGFR) and the tight junction proteins claudin-1 (CLDN1) and occludin (OCLN), which are thought to function at later stages of the HCV entry process. Using single particle imaging of HCV infection of polarized hepatoma spheroids, we observed that EGFR performs multiple functions in HCV entry, both phosphorylation-dependent and -independent. We previously observed, and in this study confirmed, that EGFR is not required for HCV migration to the tight junction. EGFR is required for the recruitment of clathrin to HCV in a phosphorylation-independent manner. EGFR phosphorylation is required for virion internalization at a stage following the recruitment of clathrin. HCV entry activates the RAF-MEK-ERK signaling pathway downstream of EGFR phosphorylation. This signaling pathway regulates the sorting and maturation of internalized HCV into APPL1- and EEA1-associated early endosomes, which form the site of virion uncoating. The tight junction proteins, CLDN1 and OCLN, function at two distinct stages of HCV entry. Despite its appreciated function as a "late receptor" in HCV entry, CLDN1 is required for efficient HCV virion accumulation at the tight junction. Huh-7.5 cells lacking CLDN1 accumulate HCV virions primarily at the initial basolateral surface. OCLN is required for the late stages of virion internalization. This study produced further insight into the unusually complex HCV endocytic process.
Copyright: © 2023 So et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
-
Homo sapiens (Human)
-
Immunology and Microbiology
In International Journal of Medical Sciences on 15 April 2023 by Lyu, X., Cai, J., et al.
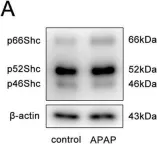
Objective: Obstructive sleep apnea (OSA) is characterized by nocturnal intermittent hypoxemia and linked to oxidative stress. Evidence demonstrated that p66Shc plays a key role in regulating oxidative stress. This study aimed to investigate the expression of p66Shc in peripheral blood mononuclear cells (PBMCs) of patients with OSA and the association with polysomnographic parameters. Methods: Fifty-four OSA subjects and 19 no OSA controls were enrolled in this study. All the subjects underwent standard polysomnography. P66Shc mRNA and protein levels in the PBMCs were detected by quantitative real-time polymerase chain reaction and western blotting. Plasma 3-nitrotyrosine (3-NT), oxidized low density lipoprotein (oxLDL), and advanced oxidation protein products (AOPP) were measured by ELISA method. Results: P66Shc mRNA and protein levels in PBMCs were significantly higher in OSA patients than in controls. P66Shc mRNA was positively correlated with plasma 3-NT, oxLDL, AOPP, hypopnea index (AHI), oxygen desaturation index (ODI), percentage of total sleep time with oxygen saturation (SaO2) below 90% (CT90), epworth sleepiness scale (ESS) and lymphocytes; negatively correlated with lowest SaO2 (LSaO2) and mean SaO2 (MSaO2). Further multivariate linear regression analysis showed that p66Shc mRNA levels were independently associated with AHI, MSaO2 and CT90. Conclusions: Oxidative stress regulator p66Shc may play a role in the pathophysiology of OSA and might serve as a potential biomarker for this disease.
© The author(s).
-
WB
-
Homo sapiens (Human)
-
Cardiovascular biology
-
Immunology and Microbiology
In Evidence-based Complementary and Alternative Medicine : eCAM on 7 October 2022 by Kim, S., Nagar, H., et al.
Breast cancer is the most common cancer and the leading cause of cancer-related mortality among females worldwide. Triple-negative breast cancer (TNBC) accounts for about 10-15% of all breast cancers and is usually more aggressive and has a poorer prognosis. Sericite has been known to have antitumor and immune-stimulatory effects. Although the chemopreventive potential of sericite has been demonstrated in other cancers, its molecular pathways in TNBC still require investigation. Thus, in the present study, the antitumor mechanism of sericite against MDA-MB231 breast cancer cells was examined in vitro and in an in vivo xenograft mouse model. Sericite treatment reduced cell proliferation and cell proliferation marker proliferating cell nuclear antigen (PCNA) in MDA-MB231 cells. It also decreased the total cell number and arrested cells in the G0/G1 phase of the cell cycle with an increase in the phosphorylation of P53 and upregulation of cell cycle regulatory proteins P21 and P16. In addition, sericite treatment also induced apoptosis signaling, which was evident by the upregulation of apoptotic protein markers cleaved caspases 3 and 9. A reduction in reactive oxygen species (ROS), NADPH oxidase 4 (NOX4), p22phox, and heat shock proteins (HSPs) was also observed. Similar results were obtained in vivo with significantly reduced tumor volume in sericite-administered mice. Collectively, these findings suggest that sericite has antitumor potential based on its property to induce cell cycle arrest and apoptotic cell death and therefore could serve as a potential therapeutic agent and crucial candidate in anticancer drug development for TNBC.
Copyright © 2022 Seonhee Kim et al.
-
WB
-
Cancer Research
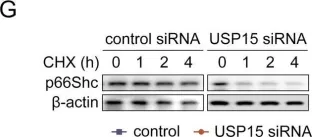
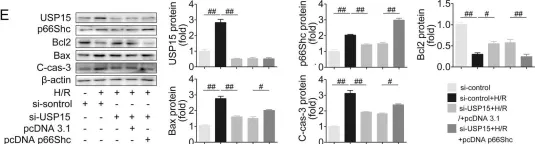
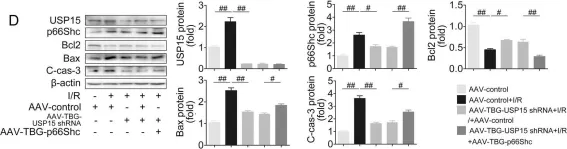
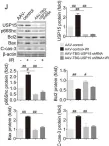
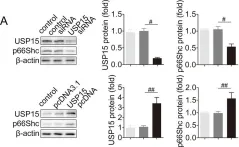
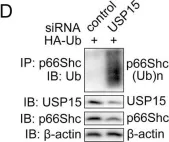
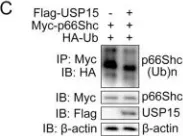
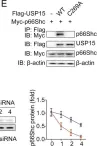
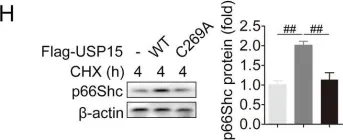
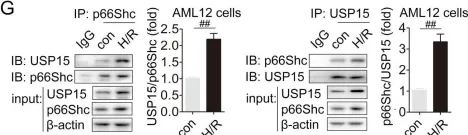
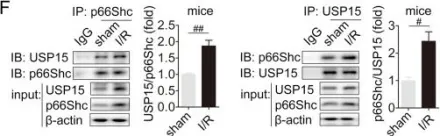
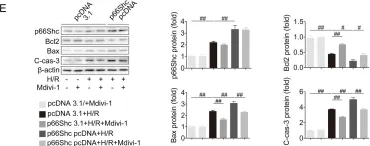
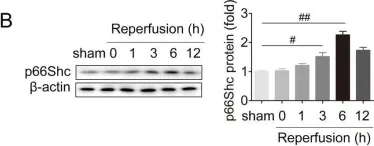
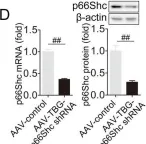
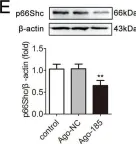
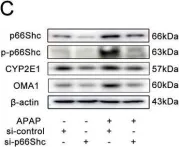
USP15 regulates p66Shc stability associated with Drp1 activation in liver ischemia/reperfusion.
In Cell Death & Disease on 26 September 2022 by Tian, X., Zhao, Y., et al.
Liver ischemia/reperfusion (I/R) injury is a major clinical concern of liver transplantation, which accounts for organ rejection and liver dysfunction. The adaptor protein p66Shc acts as a crucial redox enzyme and is implicated in liver I/R. Elevated p66Shc expression is associated with hepatocellular apoptosis in liver I/R, but the molecular mechanisms of p66Shc responsible for its aberrant expression and function remain unknown. In the present study, hepatocyte-specific p66Shc-knockdown mice exhibited clear inhibition in hepatocellular apoptosis and oxidative stress under liver I/R, while hepatocyte-specific p66Shc overexpression mice displayed the deteriorative impairment. Mechanistically, p66Shc-triggered mitochondrial fission and apoptosis in liver I/R by mediating ROS-driven Drp1 activation. Furthermore, a screening for p66Shc-interacting proteins identified ubiquitin-specific protease 15 (USP15) as a mediator critical for abnormal p66Shc expression. Specifically, USP15 interacted with the SH2 domain of p66Shc and maintained its stabilization by removing ubiquitin. In vivo, p66Shc knockdown abrogated USP15-driven hepatocellular apoptosis, whereas p66Shc overexpression counteracted the antiapoptotic effect of USP15 silencing in response to liver I/R. There was clinical evidence for the positive association between p66Shc and USP15 in patients undergoing liver transplantation. In summary, p66Shc contributes to mitochondrial fission and apoptosis associated with Drp1 activation, and abnormal p66Shc expression relies on the activity of USP15 deubiquitination under liver I/R. The current study sheds new light on the molecular mechanism of p66Shc, and identifies USP15 as a novel mediator of p66Shc to facilitate better therapeutics against liver I/R.
© 2022. The Author(s).
-
WB
-
Mus musculus (House mouse)
-
Cell Biology
In Cell Death Dis on 26 September 2022 by Tian, X., Zhao, Y., et al.
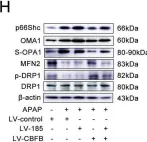
Fig.4.G

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 30
In Cell Death Dis on 26 September 2022 by Tian, X., Zhao, Y., et al.
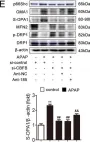
Fig.7.E

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 30
In Cell Death Dis on 26 September 2022 by Tian, X., Zhao, Y., et al.
Fig.7.D

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 30
In Cell Death Dis on 26 September 2022 by Tian, X., Zhao, Y., et al.
Fig.5.K

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 30
In Cell Death Dis on 26 September 2022 by Tian, X., Zhao, Y., et al.
Fig.5.J

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 30
In Cell Death Dis on 26 September 2022 by Tian, X., Zhao, Y., et al.
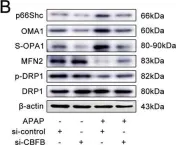
Fig.4.A

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 30
In Cell Death Dis on 26 September 2022 by Tian, X., Zhao, Y., et al.
Fig.4.D

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 30
In Cell Death Dis on 26 September 2022 by Tian, X., Zhao, Y., et al.
Fig.4.C

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 30
In Cell Death Dis on 26 September 2022 by Tian, X., Zhao, Y., et al.
Fig.4.E

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 30
In Cell Death Dis on 26 September 2022 by Tian, X., Zhao, Y., et al.
Fig.4.H

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 30
In Cell Death Dis on 26 September 2022 by Tian, X., Zhao, Y., et al.
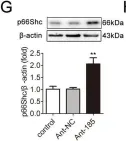
Fig.3.G

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 30
In Cell Death Dis on 26 September 2022 by Tian, X., Zhao, Y., et al.
Fig.3.H

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 30
In Cell Death Dis on 26 September 2022 by Tian, X., Zhao, Y., et al.
Fig.3.L

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 30
In Cell Death Dis on 26 September 2022 by Tian, X., Zhao, Y., et al.
Fig.2.E

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 30
In Cell Death Dis on 26 September 2022 by Tian, X., Zhao, Y., et al.
Fig.1.B

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 30
In Cell Death Dis on 26 September 2022 by Tian, X., Zhao, Y., et al.
Fig.1.D

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 30
In Cell Death Dis on 6 November 2020 by Wang, Z., Zhao, Y., et al.
Fig.4.E

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 30
In Cell Death Dis on 6 November 2020 by Wang, Z., Zhao, Y., et al.
Fig.8.H

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 30
In Cell Death Dis on 6 November 2020 by Wang, Z., Zhao, Y., et al.
Fig.7.E

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 30
In Cell Death Dis on 6 November 2020 by Wang, Z., Zhao, Y., et al.
Fig.7.B

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 30
In Cell Death Dis on 6 November 2020 by Wang, Z., Zhao, Y., et al.
Fig.4.G

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 30
In Cell Death Dis on 6 November 2020 by Wang, Z., Zhao, Y., et al.
Fig.2.C

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 30
In Cell Death Dis on 6 November 2020 by Wang, Z., Zhao, Y., et al.
Fig.1.A

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 30
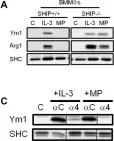
In PLoS One on 16 December 2016 by Ho, V. W., Hofs, E., et al.
Fig.1.D,F

-
WB
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 30
In PLoS One on 16 December 2016 by Ho, V. W., Hofs, E., et al.
Fig.1.A,C

-
WB
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 30