Coordinated cell cycle regulation is essential for homeostasis, with most cells in the body residing in quiescence (G0). Many pathologies arise due to disruptions in tissue-specific G0, yet little is known about the temporal-spatial mechanisms that establish G0 and its signaling hub, primary cilia. Mechanistic insight is limited by asynchronous model systems and failure to connect context-specific, transient mechanisms to function. To address this gap, we developed STAMP (synchronized temporal-spatial analysis via microscopy and phosphoproteomics) to track changes in cellular landscape occurring throughout G0 transition and ciliogenesis. We synchronized ciliogenesis and G0 transition in two cell models and combined microscopy with phosphoproteomics to order signals for further targeted analyses. We propose that STAMP is broadly applicable for studying temporal-spatial signaling in many biological contexts. The findings revealed through STAMP provide critical insight into healthy cellular functions often disrupted in pathologies, paving the way for targeted therapeutics.
Product Citations: 34
Synchronized temporal-spatial analysis via microscopy and phosphoproteomics (STAMP) of quiescence.
In Science Advances on 25 April 2025 by Azizzanjani, M. O., Turn, R. E., et al.
Dendritic cell phagosomes recruit GRASP55 for export of antigen-loaded MHC molecules.
In Cell Reports on 25 February 2025 by Cebrian, I., Dinamarca, S., et al.
Dendritic cells (DCs) present exogenous antigens via major histocompatibility complex class I (MHC-I) and MHC class II (MHC-II) molecules, activating CD8+ and CD4+ T cells. A critical but poorly understood step in this process is the trafficking of peptide-loaded MHC molecules from the endocytic system to the cell surface. In this study, we demonstrate that the Golgi reassembly-stacking protein of 55 kDa (GRASP55), which has been shown to have no role in stacking, is essential for antigen presentation. Using soluble, bead-coated, and bacterial-bound antigens, we found significantly impaired exogenous antigen presentation in GRASP55-deficient bone-marrow-derived DCs (BMDCs). Notably, GRASP55 was recruited to late phagosomes, and our data suggest that it is crucial for sorting MHC-I and MHC-II molecules, facilitating their trafficking to the plasma membrane. Our findings highlight the vital role of GRASP55 in the intracellular transport of MHC molecules bound to their respective peptides during exogenous antigen presentation.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
-
Immunology and Microbiology
In Nature Communications on 4 February 2025 by Kondo, N., Mimori-Kiyosue, Y., et al.
The leukocyte integrin LFA1 is indispensable for immune responses, orchestrating lymphocyte trafficking and adhesion. While LFA1 activation induces LFA1 clustering at the cell contact surface via outside-in signaling, the regulatory mechanisms remain unclear. Here, we uncovered a previously hidden function of the autophagosome component LC3 beyond its role in autophagy by bridging two seemingly unrelated pathways: LFA1 transport and autophagosome transport. LFA1 clusters co-trafficked with LC3, facilitating LFA1 accumulation at the contact surface. LC3b knockout decreased lymphocyte adhesiveness. LFA1 activation did not induce autophagy, whereas it increased mTOR and AMPK activity. LFA1-dependent AMPK activation enhances LFA1 and LC3 clustering and adhesion. Inhibiting Mst1 kinase-mediated LC3 phosphorylation promoted LC3-mediated LFA1 recruitment to the contact surface through direct interaction with RAPL, uncovering an unprecedented integrin recruitment route. These findings uncover a function of LC3 and expand our understanding of lymphocyte regulation via LFA1.
© 2025. The Author(s).
-
Rattus norvegicus (Rat)
-
Cell Biology
Collapse of late endosomal pH elicits a rapid Rab7 response via the V-ATPase and RILP.
In Journal of Cell Science on 1 May 2024 by Mulligan, R. J., Magaj, M. M., et al.
Endosomal-lysosomal trafficking is accompanied by the acidification of endosomal compartments by the H+-V-ATPase to reach low lysosomal pH. Disruption of the correct pH impairs lysosomal function and the balance of protein synthesis and degradation (proteostasis). Here, we treated mammalian cells with the small dipeptide LLOMe, which is known to permeabilize lysosomal membranes, and find that LLOMe also impacts late endosomes (LEs) by neutralizing their pH without causing membrane permeabilization. We show that LLOMe leads to hyperactivation of Rab7 (herein referring to Rab7a), and disruption of tubulation and mannose-6-phosphate receptor (CI-M6PR; also known as IGF2R) recycling on pH-neutralized LEs. pH neutralization (NH4Cl) and expression of Rab7 hyperactive mutants alone can both phenocopy the alterations in tubulation and CI-M6PR trafficking. Mechanistically, pH neutralization increases the assembly of the V1G1 subunit (encoded by ATP6V1G1) of the V-ATPase on endosomal membranes, which stabilizes GTP-bound Rab7 via RILP, a known interactor of Rab7 and V1G1. We propose a novel pathway by which V-ATPase and RILP modulate LE pH and Rab7 activation in concert. This pathway might broadly contribute to pH control during physiologic endosomal maturation or starvation and during pathologic pH neutralization, which occurs via lysosomotropic compounds and in disease states.
© 2024. Published by The Company of Biologists Ltd.
-
Cell Biology
In Cell Reports on 26 March 2024 by Neukam, M., Sala, P., et al.
Endocrine cells employ regulated exocytosis of secretory granules to secrete hormones and neurotransmitters. Secretory granule exocytosis depends on spatiotemporal variables such as proximity to the plasma membrane and age, with newly generated granules being preferentially released. Despite recent advances, we lack a comprehensive view of the molecular composition of insulin granules and associated changes over their lifetime. Here, we report a strategy for the purification of insulin secretory granules of distinct age from insulinoma INS-1 cells. Tagging the granule-resident protein phogrin with a cleavable CLIP tag, we obtain intact fractions of age-distinct granules for proteomic and lipidomic analyses. We find that the lipid composition changes over time, along with the physical properties of the membrane, and that kinesin-1 heavy chain (KIF5b) as well as Ras-related protein 3a (RAB3a) associate preferentially with younger granules. Further, we identify the Rho GTPase-activating protein (ARHGAP1) as a cytosolic factor associated with insulin granules.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
-
Endocrinology and Physiology
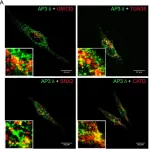
In PLoS One on 9 March 2017 by da Silva, E. Z., Freitas-Filho, E. G., et al.
Fig.2.A

-
ICC-IF
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 1