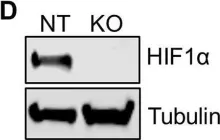
Accumulation of misfolded α-synuclein protein in intracellular inclusion bodies of dopaminergic neurons underlies the pathogenesis of Synucleinopathies, which include Parkinson’s Disease (PD), Dementia with Lewy Bodies (DLB) and Multiple System Atrophy (MSA). Therefore, clearance of misfolded α-synuclein from dopaminergic neurons could in principle offer a therapeutic window for Synucleinopathies, which currently remain untreatable. In this study, we employ the Affinity-directed PROtein Missile (AdPROM) system consisting of the substrate receptor of the CUL2-E3 ligase complex VHL and a nanobody selectively recognising the human α-synuclein protein and demonstrate targeted degradation of endogenous α-synuclein from human cell lines with remarkable selectivity. We further demonstrate that targeted degradation of α-synuclein prevents the pre-formed fibril (PFF)-induced aggregation of α-synuclein in primary neurons derived from rats expressing human α-synuclein. This approach represents the first demonstration of nanobody-guided proteasomal degradation of all clinically relevant α-synuclein variants, highlighting its potential as a therapeutic strategy against Synucleinopathies.
Product Citations: 366
Targeted degradation of α-synuclein prevents PFF-induced aggregation
Preprint on BioRxiv : the Preprint Server for Biology on 19 April 2025 by Carton, B., Gelders, G., et al.
-
WB
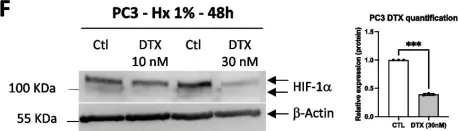
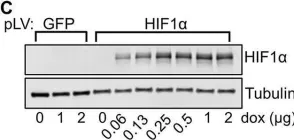
Iron content of glioblastoma tumours and role of ferrous iron in the hypoxic response in vitro.
In Frontiers in Oncology on 24 March 2025 by Praditi, C., Beverley-Stone, E., et al.
Glioblastomas are an aggressive primary brain cancer, characterised by hypoxia and poor patient survival. Iron is the most abundant transition metal in the brain, yet data on the iron content of brain cancers is sparse. Ferrous iron is an essential cofactor for a super-family of enzymes, the iron- and 2-oxoglutarate-dependent dioxygenase enzymes (2-OGDD). These enzymes control the response to hypoxia via hydroxylation of the hypoxia-inducible factor-1α (HIF-1α), and DNA demethylation via hydroxylation of 5-methyl cytosines (5hmC).
This study used clinical glioblastoma samples from 40 patients to determine the relationship between 2-OGDD activity and iron. Elemental iron was measured using inductively coupled plasma mass spectrometry (ICP-MS) and ferrous iron was measured using the colorimetric ferrozine assay. Iron measurements were compared against patient survival and clinicopathological data, and 2-OGDD-dependent activity of HIF-1 activation and 5hmC.
Elemental and ferrous iron levels were weakly related. Higher ferrous iron content of clinical glioblastoma tissue was associated with longer overall survival compared to lower ferrous iron content, but elemental iron showed no such relationship. Neither form of iron was related to clinicopathological data or markers of 2-OGDD activity. The impact of iron supplementation on the hypoxic response was assessed in three glioblastoma cell lines in vitro, similarly showing only a limited influence of iron on these 2-OGDD enzymes. Our data, together with prior studies in anaemic patients, highlight the importance of healthy iron levels in patients with glioblastoma, but further mechanistic studies are needed to elucidate the molecular pathways involved.
Copyright © 2025 Praditi, Beverley-Stone, Reid, Burgess, Crake, Vissers, Royds, Slatter, Dachs and Phillips.
-
WB
-
Homo sapiens (Human)
-
Cancer Research
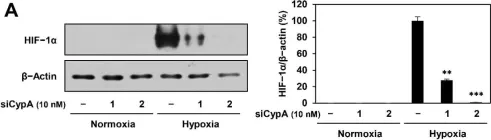
In The Journal of Biological Chemistry on 1 March 2025 by Zhang, B., Zhu, Y., et al.
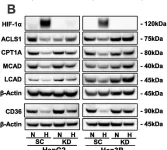
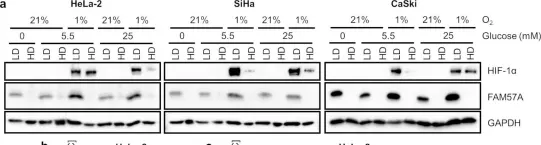
Hypoxia, a hallmark of solid tumors, is associated with increased lipid droplet (LD) accumulation. However, the mechanisms underlying this remain elusive. Here, we identify Mediator complex subunit 15 (MED15) as a critical regulator of hypoxia-inducible factor (HIF) signaling, potentially impacting LD accumulation. In mammalian cells, we elucidated that MED15, as a HIF target gene, participates in promoting HIF transcriptional activity without affecting HIFα protein levels, creating a positive feedback loop. Furthermore, zebrafish deficiency in med15 displayed decreased HIF activity and impaired tolerance to hypoxic stress. Functionally, MED15 deficiency attenuated the proliferation of colon and renal cancer cells in vitro and tumor growth in vivo. Mechanistically, MED15 acts upstream of carnitine palmitoyltransferase 1A (CPT1A), a key enzyme in fatty acid oxidation, ultimately promoting HIF-mediated LD accumulation. Disrupting the MED15-CPT1A axis impairs this process. These findings reveal a novel MED15-HIF-CPT1A axis that promotes LD formation, potentially contributing to hypoxic tumor progression.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
-
Biochemistry and Molecular biology
-
Cancer Research
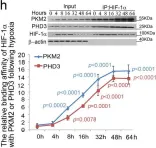
In Nature Communications on 10 February 2025 by Ramage, D. E., Grant, D. W., et al.
The 26S proteasome is a multi-catalytic protease that serves as the endpoint for protein degradation via the ubiquitin-proteasome system. Proteasome function requires the concerted activity of 33 distinct gene products, but how the expression of proteasome subunits is regulated in mammalian cells remains poorly understood. Leveraging coessentiality data from the DepMap project, here we characterize an essential role for the dystonia gene THAP1 in maintaining the basal expression of PSMB5. PSMB5 insufficiency resulting from loss of THAP1 leads to defects in proteasome assembly, impaired proteostasis and cell death. Exploiting the fact that the toxicity associated with loss of THAP1 can be rescued upon exogenous expression of PSMB5, we define the transcriptional targets of THAP1 through RNA-seq analysis and perform a deep mutational scan to systematically assess the function of thousands of single amino acid THAP1 variants. Altogether, these data identify THAP1 as a critical regulator of proteasome function and suggest that aberrant proteostasis may contribute to the pathogenesis of THAP1 dystonia.
© 2025. The Author(s).
-
Homo sapiens (Human)
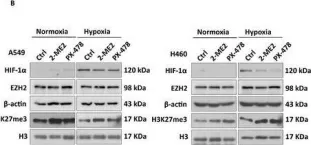
In The FEBS Journal on 1 February 2025 by Li, H. H., Hung, H. Y., et al.
Hypoxia is a critical microenvironmental factor that induces tumorigenesis and cancer progression, including metastasis. The highly dynamic nature of the extracellular matrix (ECM) plays a crucial role in metastasis. Collagens are the predominant component of structural proteins embedded within the ECM. The biosynthesis of collagen typically undergoes a series of posttranslational modifications, such as hydroxylation of lysine and proline residues by procollagen-lysine, 2-oxoglutarate 5-dioxygenases (PLODs) and prolyl 4-hydroxylases (P4Hs), respectively. Collagen hydroxylation is critical for ECM remodeling and maintenance. We recently investigated hypoxia-induced translation in human colon cancer HCT116 cells and identified several collagen-modifying enzymes, including procollagen-lysine, 2-oxoglutarate 5-dioxygenase 2 (PLOD2) and prolyl 4-hydroxylase subunit alpha 1 (P4HA1). Although the translation of bulk mRNAs is repressed in hypoxia, specific mRNAs remain efficiently translated under such conditions. We have found that PLOD2 and P4HA1 are significantly upregulated in hypoxic HCT116 cells compared to normoxic cells. HIF-1 is known to induce the transcription of PLOD2 and P4HA1 during hypoxia. However, the molecular mechanisms of hypoxia-induced translation of PLOD2 and P4HA1 remain largely unclear. We provide evidence that RBM4 and eIF4E2 are required for hypoxia-induced translation of PLOD2 and P4HA1 mRNAs. The 3' UTRs of PLOD2 and P4HA1 mRNAs are involved in translational control during hypoxia in HCT116 cells.
© 2024 Federation of European Biochemical Societies.
-
WB
-
Biochemistry and Molecular biology
-
Cancer Research
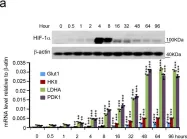
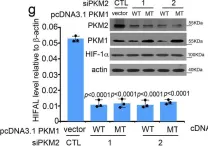
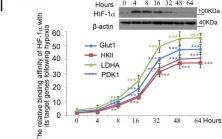
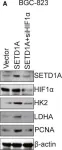
In Cell Mol Biol Lett on 8 July 2024 by Peng, S., Guo, Y., et al.
Fig.5.F

-
WB
-
Homo sapiens (Human)
Collected and cropped from Cell Mol Biol Lett by CiteAb, provided under a CC-BY license
Image 1 of 123
In Cell Mol Biol Lett on 8 July 2024 by Peng, S., Guo, Y., et al.
Fig.2.A,B,C

-
WB
-
Homo sapiens (Human)
Collected and cropped from Cell Mol Biol Lett by CiteAb, provided under a CC-BY license
Image 1 of 123
In Cell Mol Biol Lett on 8 July 2024 by Peng, S., Guo, Y., et al.
Fig.6.B

-
WB
-
Homo sapiens (Human)
Collected and cropped from Cell Mol Biol Lett by CiteAb, provided under a CC-BY license
Image 1 of 123
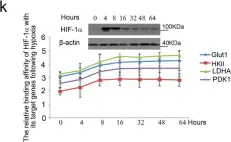
In Cell Stress on 13 March 2024 by Lin, Z., Roche, M. E., et al.
Fig.4.D

-
WB
-
Collected and cropped from Cell Stress by CiteAb, provided under a CC-BY license
Image 1 of 123
In Adv Sci (Weinh) on 1 February 2024 by Wang, J., Yang, C., et al.
Fig.1.B

-
WB
-
Collected and cropped from Adv Sci (Weinh) by CiteAb, provided under a CC-BY license
Image 1 of 123
In Adv Sci (Weinh) on 1 February 2024 by Wang, J., Yang, C., et al.
Fig.1.D

-
WB
-
Collected and cropped from Adv Sci (Weinh) by CiteAb, provided under a CC-BY license
Image 1 of 123
In Cancer Metab on 8 December 2023 by Matsufuji, S., Kitajima, Y., et al.
Fig.5.B

-
WB
-
Collected and cropped from Cancer Metab by CiteAb, provided under a CC-BY license
Image 1 of 123
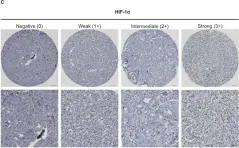
In Sci Rep on 18 July 2023 by Karakaya, S., Gunnesson, L., et al.
Fig.1.C

-
IHC
-
Homo sapiens (Human)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 123
In Cell Death Dis on 10 June 2023 by Domingo-Reinés, J., Montes, R., et al.
Fig.3.E

-
WB
-
Homo sapiens (Human)
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 123
In Cells on 21 October 2022 by Yang, D., Strobel, T. D., et al.
Fig.3.A

-
WB
-
Collected and cropped from Cells by CiteAb, provided under a CC-BY license
Image 1 of 123
In Cells on 21 October 2022 by Yang, D., Strobel, T. D., et al.
Fig.1.A

-
WB
-
Collected and cropped from Cells by CiteAb, provided under a CC-BY license
Image 1 of 123
In MBio on 23 March 2021 by Wise, L. M., Xi, Y., et al.
Fig.1.A

-
WB
-
Collected and cropped from MBio by CiteAb, provided under a CC-BY license
Image 1 of 123
In MBio on 23 March 2021 by Wise, L. M., Xi, Y., et al.
Fig.1.D

-
WB
-
Collected and cropped from MBio by CiteAb, provided under a CC-BY license
Image 1 of 123
In MBio on 23 March 2021 by Wise, L. M., Xi, Y., et al.
Fig.3.C

-
WB
-
Collected and cropped from MBio by CiteAb, provided under a CC-BY license
Image 1 of 123
In Int J Mol Sci on 1 March 2021 by Han, J. M., Sohng, J. K., et al.
Fig.9.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 123
In Nat Commun on 26 February 2021 by Zheng, F., Chen, J., et al.
Fig.6.H

-
WB
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 123
In Nat Commun on 26 February 2021 by Zheng, F., Chen, J., et al.
Fig.1.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 123
In Nat Commun on 26 February 2021 by Zheng, F., Chen, J., et al.
Fig.5.G

-
WB
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 123
In Nat Commun on 26 February 2021 by Zheng, F., Chen, J., et al.
Fig.1.I

-
WB
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 123
In Nat Commun on 26 February 2021 by Zheng, F., Chen, J., et al.
Fig.1.K

-
WB
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 123
In Int J Mol Sci on 15 December 2020 by Zhuang, L., Ly, R., et al.
Fig.1.B

-
WB
-
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 123
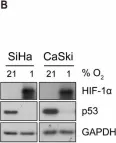
In Int J Mol Sci on 10 December 2020 by Bouthelier, A., Meléndez-Rodríguez, F., et al.
Fig.1.B

-
WB
-
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 123
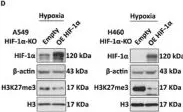
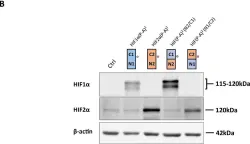
In Mov Disord on 1 December 2020 by Heras-Garvin, A., Danninger, C., et al.
Fig.1.B

-
WB
-
Homo sapiens (Human)
Collected and cropped from Mov Disord by CiteAb, provided under a CC-BY license
Image 1 of 123
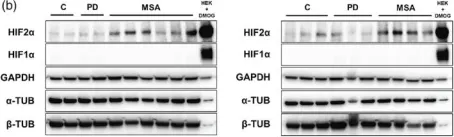
In Mol Oncol on 1 June 2020 by Wu, J., Chai, H., et al.
Fig.5.A

-
WB
-
Collected and cropped from Mol Oncol by CiteAb, provided under a CC-BY license
Image 1 of 123
In Mol Oncol on 1 June 2020 by Wu, J., Chai, H., et al.
Fig.5.C

-
WB
-
Collected and cropped from Mol Oncol by CiteAb, provided under a CC-BY license
Image 1 of 123