The Sex-linked orange mutation in domestic cats causes variegated patches of reddish/yellow hair and is a defining signature of random X-inactivation in female tortoiseshell and calico cats. Unlike the situation for most coat color genes, there is no apparent homolog for Sex-linked orange in other mammals. We show that the Sex-linked orange is caused by a 5 kb deletion that leads to ectopic and melanocyte-specific expression of the Rho GTPase Activating Protein 36 ( Arhgap36 ) gene. Single cell RNA-seq studies from fetal cat skin reveal that red/yellow hair color is caused by reduced expression of melanogenic genes that are normally activated by the Melanocortin 1 receptor (Mc1r)—cyclic adenosine monophosphate (cAMP)—protein kinase A (PKA) pathway, but the Mc1r gene and its ability to stimulate cAMP accumulation is intact. Instead, we show that increased expression of Arhgap36 in melanocytes leads to reduced levels of the PKA catalytic subunit (PKA C ); thus, Sex-linked orange is genetically and biochemically downstream of Mc1r . Our findings solve a comparative genomic conundrum, provide in vivo evidence for the ability of Arhgap36 to inhibit PKA, and reveal a molecular explanation for a charismatic color pattern with a rich genetic history.
Product Citations: 35
Molecular and genetic characterization of sex-linked orange coat color in the domestic cat
Preprint on BioRxiv : the Preprint Server for Biology on 22 November 2024 by Kaelin, C. B., McGowan, K. A., et al.
-
Genetics
-
Veterinary Research
In International Journal of Molecular Sciences on 11 July 2024 by Liu, S. H., Lin, F. J., et al.
Sleep deprivation (SD) is a recognized risk factor for atrial fibrillation (AF), yet the precise molecular and electrophysiological mechanisms behind SD-induced AF are unclear. This study explores the electrical and structural changes that contribute to AF in chronic partial SD. We induced chronic partial SD in Wistar rats using a modified multiple-platform method. Echocardiography demonstrated impaired systolic and diastolic function in the left ventricle (LV) of the SD rats. The SD rats exhibited an elevated heart rate and a higher low-frequency to high-frequency ratio in a heart-rate variability analysis. Rapid transesophageal atrial pacing led to a higher incidence of AF and longer mean AF durations in the SD rats. Conventional microelectrode recordings showed accelerated pulmonary vein (PV) spontaneous activity in SD rats, along with a heightened occurrence of delayed after-depolarizations in the PV and left atrium (LA) induced by tachypacing and isoproterenol. A Western blot analysis showed reduced expression of G protein-coupled receptor kinase 2 (GRK2) in the LA of the SD rats. Chronic partial SD impairs LV function, promotes AF genesis, and increases PV and LA arrhythmogenesis, potentially attributed to sympathetic overactivity and reduced GRK2 expression. Targeting GRK2 signaling may offer promising therapeutic avenues for managing chronic partial SD-induced AF. Future investigations are mandatory to investigate the dose-response relationship between SD and AF genesis.
-
Rattus norvegicus (Rat)
-
Cardiovascular biology
Phosphorylation of the compartmentalized PKA substrate TAF15 regulates RNA-protein interactions.
In Cellular and Molecular Life Sciences : CMLS on 3 April 2024 by Feichtner, A., Enzler, F., et al.
Spatiotemporal-controlled second messengers alter molecular interactions of central signaling nodes for ensuring physiological signal transmission. One prototypical second messenger molecule which modulates kinase signal transmission is the cyclic-adenosine monophosphate (cAMP). The main proteinogenic cellular effectors of cAMP are compartmentalized protein kinase A (PKA) complexes. Their cell-type specific compositions precisely coordinate substrate phosphorylation and proper signal propagation which is indispensable for numerous cell-type specific functions. Here we present evidence that TAF15, which is implicated in the etiology of amyotrophic lateral sclerosis, represents a novel nuclear PKA substrate. In cross-linking and immunoprecipitation experiments (iCLIP) we showed that TAF15 phosphorylation alters the binding to target transcripts related to mRNA maturation, splicing and protein-binding related functions. TAF15 appears to be one of multiple PKA substrates that undergo RNA-binding dynamics upon phosphorylation. We observed that the activation of the cAMP-PKA signaling axis caused a change in the composition of a collection of RNA species that interact with TAF15. This observation appears to be a broader principle in the regulation of molecular interactions, as we identified a significant enrichment of RNA-binding proteins within endogenous PKA complexes. We assume that phosphorylation of RNA-binding domains adds another layer of regulation to binary protein-RNAs interactions with consequences to RNA features including binding specificities, localization, abundance and composition.
© 2024. The Author(s).
-
Biochemistry and Molecular biology
-
Genetics
In Science Advances on 23 February 2024 by Omar, M. H., Byrne, D. P., et al.
Adrenal Cushing's syndrome is a disease of cortisol hypersecretion often caused by mutations in protein kinase A catalytic subunit (PKAc). Using a personalized medicine screening platform, we discovered a Cushing's driver mutation, PKAc-W196G, in ~20% of patient samples analyzed. Proximity proteomics and photokinetic imaging reveal that PKAcW196G is unexpectedly distinct from other described Cushing's variants, exhibiting retained association with type I regulatory subunits (RI) and their corresponding A kinase anchoring proteins (AKAPs). Molecular dynamics simulations predict that substitution of tryptophan-196 with glycine creates a 653-cubic angstrom cleft between the catalytic core of PKAcW196G and type II regulatory subunits (RII), but only a 395-cubic angstrom cleft with RI. Endocrine measurements show that overexpression of RIα or redistribution of PKAcW196G via AKAP recruitment counteracts stress hormone overproduction. We conclude that a W196G mutation in the kinase catalytic core skews R subunit selectivity and biases AKAP association to drive Cushing's syndrome.
Preprint on BioRxiv : the Preprint Server for Biology on 28 June 2023 by Lauer, S. M., Omar, M. H., et al.
ABSTRACT The DNAJ-PKAc fusion kinase is a defining feature of the adolescent liver cancer fibrolamellar carcinoma (FLC). A single lesion on chromosome 19 generates this mutant kinase by creating a fused gene encoding the chaperonin binding domain of Hsp40 (DNAJ) in frame with the catalytic core of protein kinase A (PKAc). FLC tumors are notoriously resistant to standard chemotherapies. Aberrant kinase activity is assumed to be a contributing factor. Yet recruitment of binding partners, such as the chaperone Hsp70, implies that the scaffolding function of DNAJ- PKAc may also underlie pathogenesis. By combining proximity proteomics with biochemical analyses and photoactivation live-cell imaging we demonstrate that DNAJ-PKAc is not constrained by A-kinase anchoring proteins. Consequently, the fusion kinase phosphorylates a unique array of substrates. One validated DNAJ-PKAc target is the Bcl-2 associated athanogene 2 (BAG2), a co-chaperone recruited to the fusion kinase through association with Hsp70. Immunoblot and immunohistochemical analyses of FLC patient samples correlate increased levels of BAG2 with advanced disease and metastatic recurrences. BAG2 is linked to Bcl-2, an anti-apoptotic factor that delays cell death. Pharmacological approaches tested if the DNAJ- PKAc/Hsp70/BAG2 axis contributes to chemotherapeutic resistance in AML12 DNAJ-PKAc hepatocyte cell lines using the DNA damaging agent etoposide and the Bcl-2 inhibitor navitoclax. Wildtype AML12 cells were susceptible to each drug alone and in combination. In contrast, AML12 DNAJ-PKAc cells were moderately affected by etoposide, resistant to navitoclax, but markedly susceptible to the drug combination. These studies implicate BAG2 as a biomarker for advanced FLC and a chemotherapeutic resistance factor in DNAJ-PKAc signaling scaffolds.
-
Cancer Research
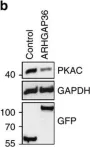
In Nat Commun on 7 October 2016 by Eccles, R. L., Czajkowski, M. T., et al.
Fig.4.C

-
WB
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 2
In Nat Commun on 7 October 2016 by Eccles, R. L., Czajkowski, M. T., et al.
Fig.4.B

-
WB
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 2