Defective apoptosis mediated by B cell lymphoma 2 antagonist/killer (BAK) or B cell lymphoma 2-associated X protein (BAX) underlies various pathologies including autoimmune and degenerative conditions. On mitochondria, voltage-dependent anion channel 2 (VDAC2) interacts with BAK and BAX through a common interface to inhibit BAK or to facilitate BAX apoptotic activity. We identified a small molecule (WEHI-3773) that inhibits interaction between VDAC2 and BAK or BAX revealing contrasting effects on their apoptotic activity. WEHI-3773 inhibits apoptosis mediated by BAX by blocking VDAC2-mediated BAX recruitment to mitochondria. Conversely, WEHI-3773 promotes BAK-mediated apoptosis by limiting inhibitory sequestration by VDAC2. In cells expressing both pro-apoptotic proteins, apoptosis promotion by WEHI-3773 dominates, because activated BAK activates BAX through a feed-forward mechanism. Loss of BAX drives resistance to the BCL-2 inhibitor venetoclax in some leukemias. WEHI-3773 overcomes this resistance by promoting BAK-mediated killing. This work highlights the coordination of BAX and BAK apoptotic activity through interaction with VDAC2 that may be targeted therapeutically.
Product Citations: 37
Differential regulation of BAX and BAK apoptotic activity revealed by small molecules.
In Science Advances on 7 March 2025 by Li, K., Yap, Y. Q., et al.
In Cell Death Discovery on 9 March 2024 by Schmitt, L., Hinxlage, I., et al.
Meriolin derivatives represent a new class of kinase inhibitors with a pronounced cytotoxic potential. Here, we investigated a newly synthesized meriolin derivative (termed meriolin 16) that displayed a strong apoptotic potential in Jurkat leukemia and Ramos lymphoma cells. Meriolin 16 induced apoptosis in rapid kinetics (within 2-3 h) and more potently (IC50: 50 nM) than the previously described derivatives meriolin 31 and 36 [1]. Exposure of Ramos cells to meriolin 16, 31, or 36 for 5 min was sufficient to trigger severe and irreversible cytotoxicity. Apoptosis induction by all three meriolin derivatives was independent of death receptor signaling but required caspase-9 and Apaf-1 as central mediators of the mitochondrial death pathway. Meriolin-induced mitochondrial toxicity was demonstrated by disruption of the mitochondrial membrane potential (ΔΨm), mitochondrial release of proapoptotic Smac, processing of the dynamin-like GTPase OPA1, and subsequent fragmentation of mitochondria. Remarkably, all meriolin derivatives were able to activate the mitochondrial death pathway in Jurkat cells, even in the presence of the antiapoptotic Bcl-2 protein. In addition, meriolins were capable of inducing cell death in imatinib-resistant K562 and KCL22 chronic myeloid leukemia cells as well as in cisplatin-resistant J82 urothelial carcinoma and 2102EP germ cell tumor cells. Given the frequent inactivation of the mitochondrial apoptosis pathway by tumor cells, such as through overexpression of antiapoptotic Bcl-2, meriolin derivatives emerge as promising therapeutic agents for overcoming treatment resistance.
© 2024. The Author(s).
-
Homo sapiens (Human)
-
Cell Biology
Suppression of Lung Cancer Malignancy by Micellized siRNA through Cell Cycle Arrest.
In Advanced Healthcare Materials on 1 April 2023 by Kim, H., Jeong, I. H., et al.
UBA6-specific E2 conjugation enzyme 1 (USE1) is frequently overexpressed in lung cancer patients. Moreover, the critical role of USE1 in the progression of human lung cancer is also indicated. As the next step, the authors aim to develop USE1-targeted therapeutic agents based on RNA interference (RNAi). In this study, a lipid-modified DNA carrier, namely U4T, which consists of four consecutive dodec-1-ynyluracil (U) nucleobases to increase the cell permeability of siRNA targeting of USE1 is introduced. The U4Ts aggregate to form micelles, and the USE1-silencing siRNA-incorporated soft spherical nucleic acid aggregate (siSNA) can be created simply through base-pairing with siRNA. Treatment with siSNA is effective in suppressing tumor growth in vivo as well as cell proliferation, migration, and invasion of lung cancer cells. Furthermore, siSNA inhibited tumor cell growth by inducing cell cycle arrest in the G1 phase and apoptosis. Thus, the anti-tumor efficacy of siSNA in lung cancer cell lines and that siSNA possesses effective cell-penetrating ability without using cationic transfection moieties are confirmed. Collectively, these results suggest that siSNA can be applied to the clinical application of RNAi-based therapeutics for lung cancer treatment.
© 2023 Wiley-VCH GmbH.
-
Cancer Research
-
Genetics
In Cell Death and Differentiation on 1 March 2023 by Huang, A. S., Chin, H. S., et al.
Intrinsic apoptosis is principally governed by the BCL-2 family of proteins, but some non-BCL-2 proteins are also critical to control this process. To identify novel apoptosis regulators, we performed a genome-wide CRISPR-Cas9 library screen, and it identified the mitochondrial E3 ubiquitin ligase MARCHF5/MITOL/RNF153 as an important regulator of BAK apoptotic function. Deleting MARCHF5 in diverse cell lines dependent on BAK conferred profound resistance to BH3-mimetic drugs. The loss of MARCHF5 or its E3 ubiquitin ligase activity surprisingly drove BAK to adopt an activated conformation, with resistance to BH3-mimetics afforded by the formation of inhibitory complexes with pro-survival proteins MCL-1 and BCL-XL. Importantly, these changes to BAK conformation and pro-survival association occurred independently of BH3-only proteins and influence on pro-survival proteins. This study identifies a new mechanism by which MARCHF5 regulates apoptotic cell death by restraining BAK activating conformation change and provides new insight into how cancer cells respond to BH3-mimetic drugs. These data also highlight the emerging role of ubiquitin signalling in apoptosis that may be exploited therapeutically.
© 2022. The Author(s), under exclusive licence to ADMC Associazione Differenziamento e Morte Cellulare.
-
FC/FACS
-
Cell Biology
In Frontiers in Oncology on 9 April 2022 by Boncompagni, G., Varone, A., et al.
An imbalance in the expression of pro- and anti-apoptotic members of the Bcl-2 family of apoptosis-regulating proteins is one of the main biological features of CLL, highlighting these proteins as therapeutic targets for treatment of this malignancy. Indeed, the Bcl-2 inhibitor Venetoclax is currently used for both first-line treatment and treatment of relapsed or refractory CLL. An alternative avenue is the transcriptional modulation of Bcl-2 family members to tilt their balance towards apoptosis. Glycerophosphoinositol (GroPIns) is a biomolecule generated from membrane phosphoinositides by the enzymes phospholipase A2 and lysolipase that pleiotropically affects key cellular functions. Mass-spectrometry analysis of GroPIns interactors recently highlighted the ability of GroPIns to bind to the non-receptor tyrosine phosphatase SHP-1, a known promoter of Bax expression, suggesting that GroPIns might correct the Bax expression defect in CLL cells, thereby promoting their apoptotic demise. To test this hypothesis, we cultured CLL cells in the presence of GroPIns, alone or in combination with drugs commonly used for treatment of CLL. We found that GroPIns alone increases Bax expression and apoptosis in CLL cells and enhances the pro-apoptotic activity of drugs used for CLL treatment in a SHP-1 dependent manner. Interestingly, among GroPIns interactors we found Bax itself. Short-term treatments of CLL cells with GroPIns induce Bax activation and translocation to the mitochondria. Moreover, GroPIns enhances the pro-apoptotic activity of Venetoclax and Fludarabine in CLL cells. These data provide evidence that GroPIns exploits two different pathways converging on Bax to promote apoptosis of leukemic cells and pave the way to new studies aimed at testing GroPIns in combination therapies for the treatment of CLL.
Copyright © 2022 Boncompagni, Varone, Tatangelo, Capitani, Frezzato, Visentin, Trentin, Corda, Baldari and Patrussi.
-
WB
-
Cancer Research
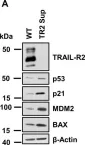
In Cell Death Dis on 31 July 2021 by Willms, A., Schupp, H., et al.
Fig.3.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 4
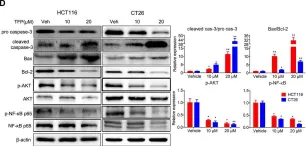
In Front Pharmacol on 2 October 2019 by Xia, Y., Jia, C., et al.
Fig.4.D

-
WB
-
Collected and cropped from Front Pharmacol by CiteAb, provided under a CC-BY license
Image 1 of 4
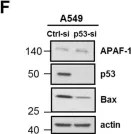
In PLoS One on 5 April 2019 by Willms, A., Schittek, H., et al.
Fig.2.T

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 4
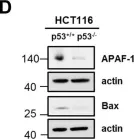
In PLoS One on 5 April 2019 by Willms, A., Schittek, H., et al.
Fig.2.D

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 4