Characterized by intracellular lipid droplet accumulation, clear cell renal cell carcinoma (ccRCC) is resistant to cytotoxic chemotherapy and is a lethal disease. Through an unbiased siRNA screen of 2-oxoglutarate (2-OG)-dependent enzymes, which play a critical role in tumorigenesis, we identified Jumonji domain-containing 6 (JMJD6) as an essential gene for ccRCC tumor development. The downregulation of JMJD6 abolished ccRCC colony formation in vitro and inhibited orthotopic tumor growth in vivo. Integrated ChIP-seq and RNA-seq analyses uncovered diacylglycerol O-acyltransferase 1 (DGAT1) as a critical JMJD6 effector. Mechanistically, JMJD6 interacted with RBM39 and co-occupied DGAT1 gene promoter with H3K4me3 to induce DGAT1 expression. JMJD6 silencing reduced DGAT1, leading to decreased lipid droplet formation and tumorigenesis. The pharmacological inhibition (or depletion) of DGAT1 inhibited lipid droplet formation in vitro and ccRCC tumorigenesis in vivo. Thus, the JMJD6-DGAT1 axis represents a potential new therapeutic target for ccRCC.
Copyright © 2022 Elsevier Inc. All rights reserved.
Product Citations: 24
In Molecular Cell on 18 August 2022 by Zhou, J., Simon, J. M., et al.
-
Biochemistry and Molecular biology
-
Cancer Research
-
Genetics
In Cell Reports on 28 December 2021 by Méndez-Solís, O., Bendjennat, M., et al.
Kaposi's sarcoma herpesvirus (KSHV) is an angiogenesis-inducing oncovirus whose ability to usurp the oxygen-sensing machinery is central to its oncogenicity. By upregulating the hypoxia-inducible factors (HIFs), KSHV reprograms infected cells to a hypoxia-like state, triggering angiogenesis. Here we identify a link between KSHV replicative biology and oncogenicity by showing that KSHV's ability to regulate HIF2α levels and localization to the endoplasmic reticulum (ER) in normoxia enables translation of viral lytic mRNAs through the HIF2α-regulated eIF4E2 translation-initiation complex. This mechanism of translation in infected cells is critical for lytic protein synthesis and contributes to KSHV-induced PDGFRA activation and VEGF secretion. Thus, KSHV regulation of the oxygen-sensing machinery allows virally infected cells to initiate translation via the mTOR-dependent eIF4E1 or the HIF2α-dependent, mTOR-independent, eIF4E2. This "translation initiation plasticity" (TRIP) is an oncoviral strategy used to optimize viral protein expression that links molecular strategies of viral replication to angiogenicity and oncogenesis.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
-
Homo sapiens (Human)
-
Biochemistry and Molecular biology
-
Cancer Research
Influenza A Virus (H1N1) Infection Induces Glycolysis to Facilitate Viral Replication.
In Virologica Sinica on 1 December 2021 by Ren, L., Zhang, W., et al.
Viruses depend on host cellular metabolism to provide the energy and biosynthetic building blocks required for their replication. In this study, we observed that influenza A virus (H1N1), a single-stranded, negative-sense RNA virus with an eight-segmented genome, enhanced glycolysis both in mouse lung tissues and in human lung epithelial (A549) cells. In detail, the expression of hexokinase 2 (HK2), the first enzyme in glycolysis, was upregulated in H1N1-infected A549 cells, and the expression of pyruvate kinase M2 (PKM2) and pyruvate dehydrogenase kinase 3 (PDK3) was upregulated in H1N1-infected mouse lung tissues. Pharmacologically inhibiting the glycolytic pathway or targeting hypoxia-inducible factor 1 (HIF-1), the central transcriptional factor critical for glycolysis, significantly reduced H1N1 replication, revealing a requirement for glycolysis during H1N1 infection. In addition, pharmacologically enhancing the glycolytic pathway further promoted H1N1 replication. Furthermore, the change of H1N1 replication upon glycolysis inhibition or enhancement was independent of interferon signaling. Taken together, these findings suggest that influenza A virus induces the glycolytic pathway and thus facilitates efficient viral replication. This study raises the possibility that metabolic inhibitors, such as those that target glycolysis, could be used to treat influenza A virus infection in the future.
© 2021. The Author(s).
-
Immunology and Microbiology
In RNA Biology on 1 November 2021 by Ma, C. N., Wo, L. L., et al.
The adaptation of tumour cells to hypoxic microenvironment is one of the most significant characteristics of many malignant tumour diseases including hepatocarcinoma. Recently, long non-coding RNAs (lncRNAs) have been reported to play important roles in the various levels of gene regulation thus functioning in growth and survival of tumour cells. Here, new hypoxia-related lncRNAs in hepatocarcinoma cells were screened and validated by lncRNA chip-array as well as real-time RT-PCR. Among them, a hypoxia-activated lncRNA that we identified and termed Hypoxia-Activated BNIP3 Overlapping Non-coding RNA (HABON), was not only regulated by hypoxic-induced factor-1α (HIF-1α) but its expression increased significantly under hypoxia in tumour cells. We deciphered the biological characteristics of HABON including its cell localization, genomic location, as well as its full-length sequence, and proved HABON could promote growth, proliferation and clone-formation of hepatocarcinoma cells under hypoxia. Then, we revealed that HABON was transcriptionally activated by HIF-1α in hypoxic cells, furthermore, it could interact with HIF-1α and promote its protein degradation, thus affecting transcription of HIF-1α's target genes to exert its effects on cells. Besides, the elevated expression of HABON under hypoxia could promote the transcriptional activation of BNIP3 through HIF-1α, and increasing the expression level of BNIP3. This research provides a novel clue for the adaptive survival and growth mechanism of tumour under hypoxia, and gives a way to reveal the nature of tumour cells' resistance characteristics to harsh microenvironment.
-
Cancer Research
-
Genetics
USP37 promotes deubiquitination of HIF2α in kidney cancer.
In Proceedings of the National Academy of Sciences of the United States of America on 9 June 2020 by Hong, K., Hu, L., et al.
Clear cell renal cell carcinoma (ccRCC) is characterized by loss of tumor suppressor Von Hippel Lindau (VHL) function, which leads to accumulation of hypoxia inducible factor α (including HIF1α and HIF2α). HIF2α was previously reported to be one of the major oncogenic drivers in ccRCC, however, its therapeutic targets remain challenging. Here we performed a deubiquitinase (DUB) complementary DNA (cDNA) library binding screen and discovered that ubiquitin-specific peptidase 37 (USP37) is a DUB that binds HIF2α and promotes HIF2α deubiquitination. As a result, USP37 promotes HIF2α protein stability in an enzymatically dependent manner, and depletion of USP37 leads to HIF2α down-regulation in ccRCC. Functionally, USP37 depletion causes decreased cell proliferation measured by MTS, two-dimensional (2D) colony formation as well as three-dimensional (3D) anchorage- independent growth. USP37 is also essential for maintaining kidney tumorigenesis in an orthotopic xenograft model and its depletion leads to both decreased primary kidney tumorigenesis and spontaneous lung metastasis. Our results suggest that USP37 is a potential therapeutic target in ccRCC.
-
Homo sapiens (Human)
-
Cancer Research
In EMBO Mol Med on 1 November 2018 by Greenhough, A., Bagley, C., et al.
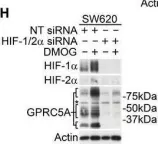
Fig.1.H

-
WB
-
Collected and cropped from EMBO Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 6
In EMBO Mol Med on 1 November 2018 by Greenhough, A., Bagley, C., et al.
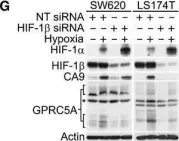
Fig.1.G

-
WB
-
Collected and cropped from EMBO Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 6
In EMBO Mol Med on 1 November 2018 by Greenhough, A., Bagley, C., et al.
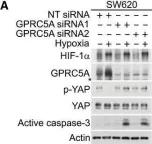
Fig.4.A

-
WB
-
Collected and cropped from EMBO Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 6
In EMBO Mol Med on 1 November 2018 by Greenhough, A., Bagley, C., et al.
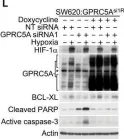
Fig.4.L

-
WB
-
Collected and cropped from EMBO Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 6
In EMBO Mol Med on 1 November 2018 by Greenhough, A., Bagley, C., et al.
Fig.4.D

-
WB
-
Collected and cropped from EMBO Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 6
In PLoS One on 5 January 2012 by Tanaka, T., Kai, S., et al.
Fig.4.A

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 6