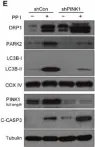
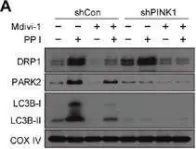
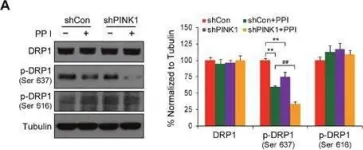
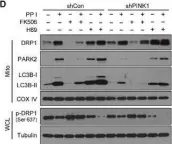
Recent studies have highlighted the importance of mitochondria in NP cells and articular chondrocyte health. Since the understanding of mechanisms governing mitochondrial dynamics in these tissues is lacking, we investigated the role of OPA1, a mitochondrial fusion protein, in their homeostasis. OPA1 knockdown in NP cells altered mitochondrial size and cristae shape and increased the oxygen consumption rate. OPA1 governed the morphology of multiple organelles, including peroxisomes, early endosomes and cis-Golgi and loss resulted in the dysregulation of autophagy. Metabolic profiling and 13C-flux analyses revealed TCA cycle anaplerosis and altered metabolism in OPA1-deficient NP cells. Noteworthy, Opa1AcanCreERT2 mice showed age-dependent disc degeneration, osteoarthritis, and vertebral osteopenia. RNA-Sequencing of Opa1cKO NP tissue revealed dysregulation of metabolism, autophagy, cytoskeletal reorganization, and extracellular matrix and shared strong thematic similarities with a subset of human degenerative NP samples. Our findings underscore that maintenance of mitochondrial dynamics and multi-organelle cross-talk is critical in preserving metabolic homeostasis of disc and cartilage.
© 2025. The Author(s).
Product Citations: 132
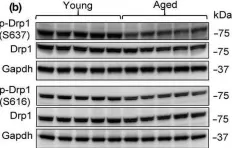
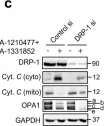
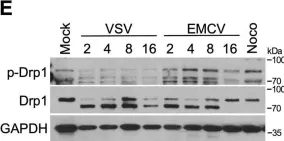
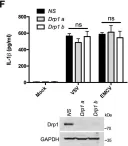
The loss of OPA1 accelerates intervertebral disc degeneration and osteoarthritis in aged mice.
In Nature Communications on 1 July 2025 by Madhu, V., Meadows, M. H., et al.
Mitochondrial mechanotransduction through MIEF1 coordinates the nuclear response to forces.
In Nature Cell Biology on 1 December 2024 by Romani, P., Benedetti, G., et al.
Tissue-scale architecture and mechanical properties instruct cell behaviour under physiological and diseased conditions, but our understanding of the underlying mechanisms remains fragmentary. Here we show that extracellular matrix stiffness, spatial confinements and applied forces, including stretching of mouse skin, regulate mitochondrial dynamics. Actomyosin tension promotes the phosphorylation of mitochondrial elongation factor 1 (MIEF1), limiting the recruitment of dynamin-related protein 1 (DRP1) at mitochondria, as well as peri-mitochondrial F-actin formation and mitochondrial fission. Strikingly, mitochondrial fission is also a general mechanotransduction mechanism. Indeed, we found that DRP1- and MIEF1/2-dependent fission is required and sufficient to regulate three transcription factors of broad relevance-YAP/TAZ, SREBP1/2 and NRF2-to control cell proliferation, lipogenesis, antioxidant metabolism, chemotherapy resistance and adipocyte differentiation in response to mechanical cues. This extends to the mouse liver, where DRP1 regulates hepatocyte proliferation and identity-hallmark YAP-dependent phenotypes. We propose that mitochondria fulfil a unifying signalling function by which the mechanical tissue microenvironment coordinates complementary cell functions.
© 2024. The Author(s).
-
Cell Biology
In Science Advances on 4 October 2024 by Seager, R., Shree, N., et al.
Mitochondria undergo fragmentation in response to bioenergetic stress, mediated by dynamin-related protein 1 (DRP1) recruitment to the mitochondria. The major pro-fission DRP1 receptor is mitochondrial fission factor (MFF), and mitochondrial dynamics proteins of 49 and 51 kilodaltons (MiD49/51), which can sequester inactive DRP1. Together, they form a trimeric DRP1-MiD-MFF complex. Adenosine monophosphate-activated protein kinase (AMPK)-mediated phosphorylation of MFF is necessary for mitochondrial fragmentation, but the molecular mechanisms are unclear. Here, we identify MFF as a target of small ubiquitin-like modifier (SUMO) at Lys151, MFF SUMOylation is enhanced following AMPK-mediated phosphorylation and that MFF SUMOylation regulates the level of MiD binding to MFF. The mitochondrial stressor carbonyl cyanide 3-chlorophenylhydrazone (CCCP) promotes MFF SUMOylation and mitochondrial fragmentation. However, CCCP-induced fragmentation is impaired in MFF-knockout mouse embryonic fibroblasts expressing non-SUMOylatable MFF K151R. These data suggest that the AMPK-MFF SUMOylation axis dynamically controls stress-induced mitochondrial fragmentation by regulating the levels of MiD in trimeric fission complexes.
-
Cell Biology
CARM1 phosphorylation at S595 by p38γ MAPK drives ROS-mediated cellular senescence.
In Redox Biology on 1 October 2024 by Cho, Y. & Kim, Y. K.
CARM1 is predominantly localized in the nucleus and plays a pivotal role in maintaining mitochondrial homeostasis by regulating gene expression. It suppresses mitochondrial biogenesis by downregulating PGC-1α and TFAM expression, while promoting mitochondrial fission through increased DNM1L expression. Under oxidative stress, CARM1 translocates to the cytoplasm, where it directly methylates DRP1 and accelerates mitochondrial fission, enhancing reactive oxygen species (ROS) production. Cytoplasmic localization of CARM1 is facilitated by its phosphorylation at S595 by ROS-activated p38γ MAPK, creating a positive feedback loop. Consequently, cytoplasmic CARM1 contributes to cellular senescence by altering mitochondrial dynamics and increasing ROS levels. This observation was supported by the increased cytoplasmic CARM1 levels and disrupted mitochondrial dynamics in the transformed 10T1/2 cells. Moreover, CARM1 inhibitors not only inhibit the proliferation of cancer cells but also induce apoptotic death in senescent cells. These findings highlight the potential of CARM1 inhibitors, particularly those targeting cytoplasmic functions, as novel strategies for eliminating cancer and senescent cells.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
mCAUSE: Prioritizing mitochondrial targets that alleviate pancreatic cancer cell phenotypes.
In IScience on 20 September 2024 by Murata, D., Ito, F., et al.
Substantial changes in energy metabolism are a hallmark of pancreatic cancer. To adapt to hypoxic and nutrient-deprived microenvironments, pancreatic cancer cells remodel their bioenergetics from oxidative phosphorylation to glycolysis. This bioenergetic shift makes mitochondria an Achilles' heel. Since mitochondrial function remains essential for pancreatic cancer cells, further depleting mitochondrial energy production is an appealing treatment target. However, identifying effective mitochondrial targets for treatment is challenging. Here, we developed an approach, mitochondria-targeted cancer analysis using survival and expression (mCAUSE), to prioritize target proteins from the entire mitochondrial proteome. Selected proteins were further tested for their impact on pancreatic cancer cell phenotypes. We discovered that targeting a dynamin-related GTPase, OPA1, which controls mitochondrial fusion and cristae, effectively suppresses pancreatic cancer activities. Remarkably, when combined with a mutation-specific KRAS inhibitor, OPA1 inhibition showed a synergistic effect. Our findings offer a therapeutic strategy against pancreatic cancer by simultaneously targeting mitochondria dynamics and KRAS signaling.
© 2024 The Author(s).
-
Cancer Research
-
Cell Biology
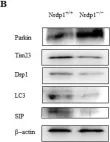
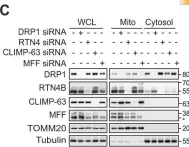
In Cells on 5 September 2023 by Luo, Z. Y., Jiang, T. X., et al.
Fig.7.B

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Cells by CiteAb, provided under a CC-BY license
Image 1 of 31
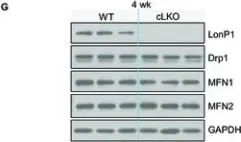
In Research (Wash D C) on 19 June 2023 by Li, Y., Huang, D., et al.
Fig.2.G

-
WB
-
Collected and cropped from Research (Wash D C) by CiteAb, provided under a CC-BY license
Image 1 of 31
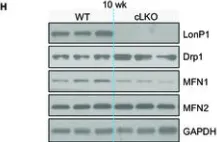
In Research (Wash D C) on 19 June 2023 by Li, Y., Huang, D., et al.
Fig.2.H

-
WB
-
Collected and cropped from Research (Wash D C) by CiteAb, provided under a CC-BY license
Image 1 of 31
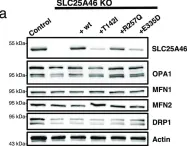
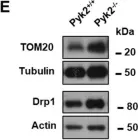
In Life Sci Alliance on 1 June 2023 by Schuettpelz, J., Janer, A., et al.
Fig.5.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Life Sci Alliance by CiteAb, provided under a CC-BY license
Image 1 of 31
In Cell Death Dis on 4 May 2022 by Carter, R. J., Milani, M., et al.
Fig.4.C

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 31
In Cells on 1 March 2022 by López-Molina, L., Fernández-Irigoyen, J., et al.
Fig.5.E

-
WB
-
Collected and cropped from Cells by CiteAb, provided under a CC-BY license
Image 1 of 31
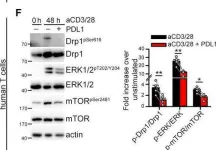
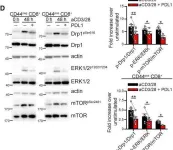
In Mol Oncol on 1 January 2022 by Simula, L., Antonucci, Y., et al.
Fig.2.F

-
WB
-
Collected and cropped from Mol Oncol by CiteAb, provided under a CC-BY license
Image 1 of 31
In Mol Oncol on 1 January 2022 by Simula, L., Antonucci, Y., et al.
Fig.2.D

-
WB
-
Collected and cropped from Mol Oncol by CiteAb, provided under a CC-BY license
Image 1 of 31
In Mol Oncol on 1 January 2022 by Simula, L., Antonucci, Y., et al.
Fig.3.C

-
WB
-
Collected and cropped from Mol Oncol by CiteAb, provided under a CC-BY license
Image 1 of 31
In Mol Oncol on 1 January 2022 by Simula, L., Antonucci, Y., et al.
Fig.3.A

-
WB
-
Collected and cropped from Mol Oncol by CiteAb, provided under a CC-BY license
Image 1 of 31
In Mol Oncol on 1 January 2022 by Simula, L., Antonucci, Y., et al.
Fig.5.D

-
WB
-
Collected and cropped from Mol Oncol by CiteAb, provided under a CC-BY license
Image 1 of 31
In Cell Death Dis on 21 October 2020 by Choi, S. Y., Lee, J. H., et al.
Fig.3.A

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 31
In Cell Death Dis on 21 October 2020 by Choi, S. Y., Lee, J. H., et al.
Fig.1.A

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 31
In Aging Cell on 1 August 2020 by Liang, W., Moyzis, A. G., et al.
Fig.6.B

-
WB
-
Collected and cropped from Aging Cell by CiteAb, provided under a CC-BY license
Image 1 of 31
In Cell Death Discov on 26 July 2019 by Milani, M., Beckett, A. J., et al.
Fig.5.C

-
WB
-
Homo sapiens (Human)
Collected and cropped from Cell Death Discov by CiteAb, provided under a CC-BY license
Image 1 of 31
In Cell Death Dis on 25 April 2019 by da Costa, L. S., Outlioua, A., et al.
Fig.5.E

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 31
In Cell Death Dis on 25 April 2019 by da Costa, L. S., Outlioua, A., et al.
Fig.5.F

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 31
In Oncotarget on 7 February 2017 by Li, G. B., Fu, R. Q., et al.
Fig.7.E

-
WB
-
Homo sapiens (Human)
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 31
In Oncotarget on 7 February 2017 by Li, G. B., Fu, R. Q., et al.
Fig.5.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 31
In Oncotarget on 7 February 2017 by Li, G. B., Fu, R. Q., et al.
Fig.6.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 31
In Oncotarget on 7 February 2017 by Li, G. B., Fu, R. Q., et al.
Fig.6.D

-
WB
-
Homo sapiens (Human)
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 31
In Oncotarget on 7 February 2017 by Li, G. B., Fu, R. Q., et al.
Fig.4.H

-
WB
-
Homo sapiens (Human)
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 31
In Oncotarget on 7 February 2017 by Li, G. B., Fu, R. Q., et al.
Fig.5.G

-
WB
-
Homo sapiens (Human)
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 31
In Oncotarget on 7 February 2017 by Li, G. B., Fu, R. Q., et al.
Fig.5.F

-
WB
-
Homo sapiens (Human)
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 31
In EMBO Mol Med on 1 September 2016 by Janer, A., Prudent, J., et al.
Fig.2.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from EMBO Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 31