The integrity of retinal endothelial cell (EC) is essential for establishing and maintaining the retinal blood barrier to ensure proper vision. Vitamin D is a hormone with known protective roles in EC function. The majority of vitamin D action is mediated through the vitamin D receptor (VDR). VDR is a nuclear receptor whose engagement by vitamin D impacts the expression of many genes with important roles in regulation of angiogenesis and inflammation. Although many studies have investigated vitamin D-VDR action in cardiovascular protection and tumor angiogenesis, its impact on retinal EC function and regulation of ocular angiogenesis and inflammation is exceedingly limited. We previously showed calcitriol, the active form of vitamin D, is a potent inhibitor of retinal neovascularization in vivo and retinal EC capillary morphogenesis in vitro. Here, using retinal EC prepared from wild-type (Vdr+/+) and VDR-deficient (Vdr-/-) mice, we show that retinal EC express VDR and its expression is induced by calcitriol. The lack of VDR expression had a significant impact on endothelial cell-cell and cell-matrix interactions. Vdr-/- retinal EC proliferated at a slower rate and were more adherent and less migratory. They also exhibited increased expression levels of inflammatory markers driven in part by sustained activation of STAT1 and NF-κB pathways and were more sensitive to oxidative challenge. These changes were attributed, in part, to down-regulation of endothelial nitric oxide synthetase, enhanced hepcidin expression, and increased intracellular iron levels. Taken together, our results indicate that VDR expression plays a fundamental role in maintaining the proper angiogenic and inflammatory state of retinal EC.
Product Citations: 16
In Cells on 16 January 2023 by Song, Y. S., Jamali, N., et al.
-
WB
-
Mus musculus (House mouse)
-
Cell Biology
-
Immunology and Microbiology
In Acta Pharmacologica Sinica on 1 January 2023 by Peng, C., Yang, L. J., et al.
It is of great clinical significance to develop potential novel strategies to prevent diabetic cardiovascular complications. Endothelial progenitor cell (EPC) dysfunction is a key contributor to diabetic vascular complications. In the present study we evaluated whether low-dose nifedipine could rescue impaired EPC-mediated angiogenesis and prevent cardiovascular complications in diabetic mice. Diabetes was induced in mice by five consecutive injections of streptozotocin (STZ, 60 mg·kg-1·d-1, i.p.). Diabetic mice were treated with low-dose nifedipine (1.5 mg·kg-1·d-1, i.g.) for six weeks. Then, circulating EPCs in the peripheral blood were quantified, and bone marrow-derived EPCs (BM-EPCs) were prepared. We showed that administration of low-dose nifedipine significantly increased circulating EPCs, improved BM-EPCs function, promoted angiogenesis, and reduced the cerebral ischemic injury in diabetic mice. Furthermore, we found that low-dose nifedipine significantly increased endothelial nitric oxide synthase (eNOS) expression and intracellular NO levels, and decreased the levels of intracellular O2.- and thrombospondin-1/2 (TSP-1/2, a potent angiogenesis inhibitor) in BM-EPCs of diabetic mice. In cultured BM-EPCs, co-treatment with nifedipine (0.1, 1 μM) dose-dependently protected against high-glucose-induced impairment of migration, and suppressed high-glucose-induced TSP-1 secretion and superoxide overproduction. In mice with middle cerebral artery occlusion, intravenous injection of diabetic BM-EPCs treated with nifedipine displayed a greater ability to promote local angiogenesis and reduce cerebral ischemic injury compared to injection of diabetic BM-EPCs treated with vehicle, and the donor-derived BM-EPCs homed to the recipient ischemic brain. In conclusion, low-dose nifedipine can enhance EPCs' angiogenic potential and protect against cerebral ischemic injury in diabetic mice. It is implied that chronic treatment with low-dose nifedipine may be a safe and economic manner to prevent ischemic diseases (including stroke) in diabetes.
© 2022. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
-
Mus musculus (House mouse)
-
Pharmacology
Endothelial Piezo1 sustains muscle capillary density and contributes to physical activity.
In The Journal of Clinical Investigation on 1 March 2022 by Bartoli, F., Debant, M., et al.
Piezo1 forms mechanically activated nonselective cation channels that contribute to endothelial response to fluid flow. Here we reveal an important role in the control of capillary density. Conditional endothelial cell-specific deletion of Piezo1 in adult mice depressed physical performance. Muscle microvascular endothelial cell apoptosis and capillary rarefaction were evident and sufficient to account for the effect on performance. There was selective upregulation of thrombospondin-2 (TSP2), an inducer of endothelial cell apoptosis, with no effect on TSP1, a related important player in muscle physiology. TSP2 was poorly expressed in muscle endothelial cells but robustly expressed in muscle pericytes, in which nitric oxide (NO) repressed the Tsp2 gene without an effect on Tsp1. In endothelial cells, Piezo1 was required for normal expression of endothelial NO synthase. The data suggest an endothelial cell-pericyte partnership of muscle in which endothelial Piezo1 senses blood flow to sustain capillary density and thereby maintain physical capability.
-
IHC
-
Mus musculus (House mouse)
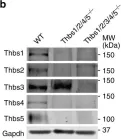
Thbs1 induces lethal cardiac atrophy through PERK-ATF4 regulated autophagy.
In Nature Communications on 24 June 2021 by Vanhoutte, D., Schips, T. G., et al.
The thrombospondin (Thbs) family of secreted matricellular proteins are stress- and injury-induced mediators of cellular attachment dynamics and extracellular matrix protein production. Here we show that Thbs1, but not Thbs2, Thbs3 or Thbs4, induces lethal cardiac atrophy when overexpressed. Mechanistically, Thbs1 binds and activates the endoplasmic reticulum stress effector PERK, inducing its downstream transcription factor ATF4 and causing lethal autophagy-mediated cardiac atrophy. Antithetically, Thbs1-/- mice develop greater cardiac hypertrophy with pressure overload stimulation and show reduced fasting-induced atrophy. Deletion of Thbs1 effectors/receptors, including ATF6α, CD36 or CD47 does not diminish Thbs1-dependent cardiac atrophy. However, deletion of the gene encoding PERK in Thbs1 transgenic mice blunts the induction of ATF4 and autophagy, and largely corrects the lethal cardiac atrophy. Finally, overexpression of PERK or ATF4 using AAV9 gene-transfer similarly promotes cardiac atrophy and lethality. Hence, we identified Thbs1-mediated PERK-eIF2α-ATF4-induced autophagy as a critical regulator of cardiomyocyte size in the stressed heart.
-
WB
-
Mus musculus (House mouse)
-
Cardiovascular biology
-
Cell Biology
Cocaine Triggers Astrocyte-Mediated Synaptogenesis.
In Biological Psychiatry on 15 February 2021 by Wang, J., Li, K. L., et al.
Synaptogenesis is essential in forming new neurocircuits during development, and this is mediated in part by astrocyte-released thrombospondins (TSPs) and activation of their neuronal receptor, α2δ-1. Here, we show that this developmental synaptogenic mechanism is utilized during cocaine experience to induce spinogenesis and the generation of AMPA receptor-silent glutamatergic synapses in the adult nucleus accumbens shell (NAcSh).
Using multidisciplinary approaches including astrocyte Ca2+ imaging, genetic mouse lines, viral-mediated gene transfer, and operant behavioral procedures, we monitor the response of NAcSh astrocytes to cocaine administration and examine the role of astrocytic TSP-α2δ-1 signaling in cocaine-induced silent synapse generation as well as the behavioral impact of astrocyte-mediated synaptogenesis and silent synapse generation.
Cocaine administration acutely increases Ca2+ events in NAcSh astrocytes, while decreasing astrocytic Ca2+ blocks cocaine-induced generation of silent synapses. Furthermore, knockout of TSP2, or pharmacological inhibition or viral-mediated knockdown of α2δ-1, prevents cocaine-induced generation of silent synapses. Moreover, disrupting TSP2-α2δ-1-mediated spinogenesis and synapse generation in NAcSh decreases cue-induced cocaine seeking after withdrawal from cocaine self-administration and cue-induced reinstatement of cocaine seeking after drug extinction.
These results establish that silent synapses are generated by an astrocyte-mediated synaptogenic mechanism in response to cocaine experience and embed critical cue-associated memory traces that promote cocaine relapse.
Copyright © 2020 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
-
Neuroscience
In Nat Commun on 8 January 2019 by Schips, T. G., Vanhoutte, D., et al.
Fig.5.B

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 1