The Hippo signaling pathway is an important regulator of organ growth and differentiation, and its deregulation contributes to the development of cancer. The activity of its downstream targets YAP/TAZ depends on adherens junctions. Plakophilin 4 (PKP4) is a cell-type specific adherens junction protein expressed in the proliferating cells of the epidermis. Here, we show that PKP4 diminishes proliferation as well as differentiation. Depletion of PKP4 increased proliferation but at the same time induced premature epidermal differentiation. PKP4 interacted with several Hippo pathway components, including the transcriptional co-activators YAP/TAZ, and promoted nuclear YAP localization and target gene expression. In differentiated keratinocytes, PKP4 recruited LATS and YAP to cell junctions where YAP is transcriptionally inactive. YAP depletion, on the other hand, reduced PKP4 levels and keratinocyte adhesion indicative of a feedback mechanism controlling adhesion, proliferation, and differentiation by balancing YAP functions.
© 2024 The Author(s).
Product Citations: 10
A feedback loop between plakophilin 4 and YAP signaling regulates keratinocyte differentiation.
In IScience on 20 September 2024 by Müller, L., Gutschner, T., et al.
Protocol to determine the subcellular localization of protein interactions in murine keratinocytes.
In STAR Protocols on 17 May 2023 by Müller, L.
Protein activities and interactions are determined by their subcellular localization. Elucidating the network of protein-protein interactions at a spatial resolution is essential for understanding the complexity of protein functions, their regulation, and cellular processes. Here, we present a protocol to determine the subcellular localization of protein interactions in non-transformed murine keratinocytes. We describe steps for nucleus/cytoplasm fractionation, immunoprecipitation from these fractions, and immunoblotting. We then detail binding quantification. For complete details on the use and execution of this protocol, please refer to Müller et al. (2023).1.
Copyright © 2023 The Author. Published by Elsevier Inc. All rights reserved.
-
Mus musculus (House mouse)
In Cell Death & Disease on 16 August 2022 by Wang, Y. L., Zhao, W. W., et al.
Paraspeckles are mammal-specific membraneless nuclear bodies that participate in various biological processes. NONO, a central paraspeckle component, has been shown to play pivotal roles in DNA double-strand breaks (DSB) repair, whereas its underlying mechanism needs to be further disclosed. Here, using co-immunoprecipitation and mass spectrum, we identified ribosomal protein P0 (RPLP0) as a DSB-induced NONO-binding protein; RPLP0 binds to the RRM1 and RRM2 domains of NONO. Similar to NONO, RPLP0 enhances non-homologous end joining-mediated DSB repair, which was ascribed to a ribosome-independent manner. Interestingly, paraspeckles were induced as early as 15 min after irradiation; it further recruited nuclear RPLP0 to enhance its interaction with NONO. Radiation-induced NONO/RPLP0 complex subsequently anchored at the damaged DNA and increased the autophosphorylation of DNA-PK at Thr2609, thereby enhancing DSB repair. Consistently, in vivo and in vitro experiments showed that depletion of NONO sensitizes tumor cells to radiation. For patients with locally advanced rectal cancer, NONO expression was remarkably increased in tumor tissues and correlated with a poor response to radiochemotherapy. Our findings suggest a pivotal role of radiation-induced paraspeckles in DNA repair and tumor radioresistance, and provide a new insight into the ribosome-independent function of ribosomal proteins.
© 2022. The Author(s).
-
WB
-
IHC
-
PLA
-
Cancer Research
-
Cell Biology
-
Genetics
In American Journal of Cancer Research on 13 July 2021 by Fan, X. J., Wang, Y. L., et al.
Radioresistance is one of the main causes of cancer treatment failure, which leads to relapse and inferior survival outcome of cancer patients. Liquid-liquid phase separation (LLPS) of proteins is known to be involved in various biological processes, whereas its role in the regulation of radiosensitivity remains largely unknown. In this study, we characterized NONO, an RNA/DNA binding protein with LLPS capacity, as an essential regulator of tumor radioresistance. In vitro assay showed that NONO involved in DNA repair via non-homologous end joining (NHEJ) manner. NONO knockout significantly reduced DNA damage repair and sensitized tumor cells to irradiation in vitro and in vivo. NONO overexpression was correlated with an inferior survival outcome in cancer patients. Mechanically, NONO was associated with nuclear EGFR (nEGFR). Both irradiation and EGF treatment induced nEGFR accumulation, thereby increased the association between NONO and nEGFR. However, NONO was not a substrate of EGFR kinase. Furthermore, NONO promoted DNA damage-induced DNA-PK phosphorylation at T2609 by enhancing the interaction between EGFR and DNA-PK. Importantly, NONO protein formed high concentration LLPS droplets in vitro, and recruited EGFR and DNA-PK. Disruption of NONO droplets with LLPS inhibitor significantly reduced the interaction between EGFR and DNA-PK, and suppressed DNA damage-induced phosphorylation of T2609-DNA-PK. Taken together, LLPS of NONO recruits nuclear EGFR and DNA-PK and enhances their interaction, further increases DNA damage-activated pT2609-DNA-PK and promotes NHEJ-mediated DNA repair, finally leads to tumor radioresistance. NONO phase separation-mediated radioresistance may serve as a novel molecular target to sensitize tumor cell to radiotherapy.
AJCR Copyright © 2021.
-
WB
-
Homo sapiens (Human)
-
Cancer Research
-
Genetics
PRMT1 enhances oncogenic arginine methylation of NONO in colorectal cancer.
In Oncogene on 1 February 2021 by Yin, X. K., Wang, Y. L., et al.
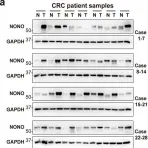
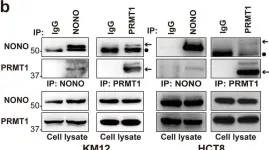
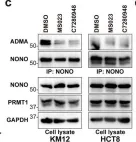
Arginine methylation is an important posttranslational modification catalyzed by protein arginine methyltransferases (PRMTs). However, the role of PRMTs in colorectal cancer (CRC) progression is not well understood. Here we report that non-POU domain-containing octamer-binding protein (NONO) is overexpressed in CRC tissue and is a potential marker for poor prognosis in CRC patients. NONO silencing resulted in decreased proliferation, migration, and invasion of CRC cells, whereas overexpression had the opposite effect. In a xenograft model, tumors derived from NONO-deficient CRC cells were smaller than those derived from wild-type (WT) cells, and PRMT1 inhibition blocked CRC xenograft progression. A mass spectrometry analysis indicated that NONO is a substrate of PRMT1. R251 of NONO was asymmetrically dimethylated by PRMT1 in vitro and in vivo. Compared to NONO WT cells, NONO R251K mutant-expressing CRC cells showed reduced proliferation, migration, and invasion, and PRMT1 knockdown or pharmacological inhibition abrogated the malignant phenotype associated with NONO asymmetric dimethylation in both KRAS WT and mutant CRC cells. Compared to adjacent normal tissue, PRMT1 was highly expressed in the CRC zone in clinical specimens, which was correlated with poor overall survival in patients with locally advanced CRC. These results demonstrate that PRMT1-mediated methylation of NONO at R251 promotes CRC growth and metastasis, and suggest that PRMT1 inhibition may be an effective therapeutic strategy for CRC treatment regardless of KRAS mutation status.
-
WB
-
IHC
-
Cancer Research
In Oncogene on 1 February 2021 by Yin, X. K., Wang, Y. L., et al.
Fig.1.A

-
WB
-
Collected and cropped from Oncogene by CiteAb, provided under a CC-BY license
Image 1 of 5
In Oncogene on 1 February 2021 by Yin, X. K., Wang, Y. L., et al.
Fig.3.B

-
WB
-
Collected and cropped from Oncogene by CiteAb, provided under a CC-BY license
Image 1 of 5
In Oncogene on 1 February 2021 by Yin, X. K., Wang, Y. L., et al.
Fig.6.C

-
WB
-
Collected and cropped from Oncogene by CiteAb, provided under a CC-BY license
Image 1 of 5
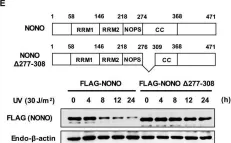
In Nucleic Acids Res on 25 January 2019 by Deshar, R., Yoo, W., et al.
Fig.4.E

-
WB
-
Collected and cropped from Nucleic Acids Res by CiteAb, provided under a CC-BY license
Image 1 of 5
In Nucleic Acids Res on 25 January 2019 by Deshar, R., Yoo, W., et al.
Fig.4.C

-
WB
-
Collected and cropped from Nucleic Acids Res by CiteAb, provided under a CC-BY license
Image 1 of 5