Accrual of ceramides, membrane and bioactive sphingolipids, has been implicated in endothelial dysfunction preceding cardiometabolic diseases. Yet, direct in vivo evidence, underlying mechanisms, and pathological implications are lacking. Here we show that suppression of ceramides and sphingosine-1-phosphate (S1P), a product of ceramide degradation, are causally linked to endothelial dysfunction and activation, contributing to vascular and metabolic disorders in high fat diet fed (HFD) male mice. Mechanistically, the upregulation of Nogo-B and ORMDL proteins suppress ceramide de novo biosynthesis in endothelial cells (EC) of HFD mice, resulting in vascular and metabolic dysfunctions. Systemic and endothelial specific deletion of Nogo-B restore sphingolipid signaling and functions, lowers hypertension, and hepatic glucose production in HFD. Our results demonstrate in vivo that ceramide and S1P suppression rather than accrual contributes to endothelial dysfunction and cardiometabolic diseases in HFD mice. Our study also sets a framework for the development of therapeutic strategies to treat these conditions.
© 2025. The Author(s).
Product Citations: 7
In Nature Communications on 25 February 2025 by Rubinelli, L., Manzo, O. L., et al.
-
WB
-
Mus musculus (House mouse)
Ceramide sensing by human SPT-ORMDL complex for establishing sphingolipid homeostasis.
In Nature Communications on 13 June 2023 by Xie, T., Liu, P., et al.
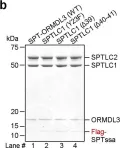
The ORM/ORMDL family proteins function as regulatory subunits of the serine palmitoyltransferase (SPT) complex, which is the initiating and rate-limiting enzyme in sphingolipid biosynthesis. This complex is tightly regulated by cellular sphingolipid levels, but the sphingolipid sensing mechanism is unknown. Here we show that purified human SPT-ORMDL complexes are inhibited by the central sphingolipid metabolite ceramide. We have solved the cryo-EM structure of the SPT-ORMDL3 complex in a ceramide-bound state. Structure-guided mutational analyses reveal the essential function of this ceramide binding site for the suppression of SPT activity. Structural studies indicate that ceramide can induce and lock the N-terminus of ORMDL3 into an inhibitory conformation. Furthermore, we demonstrate that childhood amyotrophic lateral sclerosis (ALS) variants in the SPTLC1 subunit cause impaired ceramide sensing in the SPT-ORMDL3 mutants. Our work elucidates the molecular basis of ceramide sensing by the SPT-ORMDL complex for establishing sphingolipid homeostasis and indicates an important role of impaired ceramide sensing in disease development.
© 2023. The Author(s).
-
WB
-
Homo sapiens (Human)
Sphingosine-1-phosphate controls endothelial sphingolipid homeostasis via ORMDL.
In EMBO Reports on 9 January 2023 by Sasset, L., Chowdhury, K. H., et al.
Disruption of sphingolipid homeostasis and signaling has been implicated in diabetes, cancer, cardiometabolic, and neurodegenerative disorders. Yet, mechanisms governing cellular sensing and regulation of sphingolipid homeostasis remain largely unknown. In yeast, serine palmitoyltransferase, catalyzing the first and rate-limiting step of sphingolipid de novo biosynthesis, is negatively regulated by Orm1 and 2. Lowering sphingolipids triggers Orms phosphorylation, upregulation of serine palmitoyltransferase activity and sphingolipid de novo biosynthesis. However, mammalian orthologs ORMDLs lack the N-terminus hosting the phosphosites. Thus, which sphingolipid(s) are sensed by the cells, and mechanisms of homeostasis remain largely unknown. Here, we identify sphingosine-1-phosphate (S1P) as key sphingolipid sensed by cells via S1PRs to maintain homeostasis. The increase in S1P-S1PR signaling stabilizes ORMDLs, restraining SPT activity. Mechanistically, the hydroxylation of ORMDLs at Pro137 allows a constitutive degradation of ORMDLs via ubiquitin-proteasome pathway, preserving SPT activity. Disrupting S1PR/ORMDL axis results in ceramide accrual, mitochondrial dysfunction, impaired signal transduction, all underlying endothelial dysfunction, early event in the onset of cardio- and cerebrovascular diseases. Our discovery may provide the molecular basis for therapeutic intervention restoring sphingolipid homeostasis.
© 2022 The Authors.
-
WB
S1P controls endothelial sphingolipid homeostasis via ORMDL
Preprint on BioRxiv : the Preprint Server for Biology on 23 October 2021 by Sasset, L., Chowdhury, K. H., et al.
Sphingolipids (SL) are both membrane building blocks and potent signaling molecules regulating a variety of cellular functions in both physiological and pathological conditions. Under normal physiology, sphingolipid levels are tightly regulated, whereas disruption of sphingolipid homeostasis and signaling has been implicated in diabetes, cancer, cardiovascular and autoimmune diseases. Yet, mechanisms governing cellular sensing of SL, and according regulation of their biosynthesis remain largely unknown. In yeast, serine palmitoyltransferase (SPT), catalyzing the first and rate limiting step of sphingolipid de novo biosynthesis, is negatively regulated by Orosomucoid 1 and 2 (Orm) proteins. Lowering sphingolipid levels triggers Orms phosphorylation, resulting in the removal of the inhibitory brake on SPT to enhance sphingolipid de novo biosynthesis. However, mammalian orthologs ORMDLs lack the N-terminus hosting the phosphosites. Thus, which sphingolipid(s) are sensed by the cells, and mechanisms of homeostasis remain largely unknown. This study is aimed at filling this knowledge gap. Here, we identify sphingosine-1-phosphate (S1P) as the key sphingolipid sensed by endothelial cells via S1PRs. The increase of S1P-S1PR signaling stabilizes ORMDLs, which downregulates SPT activity to maintain SL homeostasis. These findings reveal the S1PR/ORMDLs axis as the sensor-effector unit regulating SPT activity accordingly. Mechanistically, the hydroxylation of ORMDLs at Pro137 allows a constitutive degradation of ORMDLs via ubiquitin-proteasome pathway, therefore preserving SPT activity at steady state. The disruption of the S1PR/ORMDL axis results in ceramide accrual, mitochondrial dysfunction, and impaired signal transduction, all leading to endothelial dysfunction, which is an early event in the onset of cardio- and cerebrovascular diseases. The disruption of S1P-ORMDL-SPT signaling may be implicated in the pathogenesis of conditions such as diabetes, cancer, cardiometabolic disorders, and neurodegeneration, all characterized by deranged sphingolipid metabolism. Our discovery may provide the molecular basis for a therapeutic intervention to restore sphingolipid homeostasis.
-
WB
Role of 1-Deoxysphingolipids in docetaxel neurotoxicity.
In Journal of Neurochemistry on 1 September 2020 by Becker, K. A., Uerschels, A. K., et al.
A major dose-limiting side effect of docetaxel chemotherapy is peripheral neuropathy. Patients' symptoms include pain, numbness, tingling and burning sensations, and motor weakness in the extremities. The molecular mechanism is currently not understood, and there are no treatments available. Previously, we have shown an association between neuropathy symptoms of patients treated with paclitaxel and the plasma levels of neurotoxic sphingolipids, the 1-deoxysphingolipids (1-deoxySL) (Kramer et al, FASEB J, 2015). 1-DeoxySL are produced when the first enzyme of the sphingolipid biosynthetic pathway, serine palmitoyltransferase (SPT), uses L-alanine as a substrate instead of its canonical amino acid substrate, L-serine. In the current investigation, we tested whether 1-deoxySL accumulate in the nervous system following systemic docetaxel treatment in mice. In dorsal root ganglia (DRG), we observed that docetaxel (45 mg/kg cumulative dose) significantly elevated the levels of 1-deoxySL and L-serine-derived ceramides, but not sphingosine-1-phosphate (S1P). S1P is a bioactive sphingolipid and a ligand for specific G-protein-coupled receptors. In the sciatic nerve, docetaxel decreased 1-deoxySL and ceramides. Moreover, we show that in primary DRG cultures, 1-deoxysphingosine produced neurite swellings that could be reversed with S1P. Our results demonstrate that docetaxel chemotherapy up-regulates sphingolipid metabolism in sensory neurons, leading to the accumulation of neurotoxic 1-deoxySL. We suggest that the neurotoxic effects of 1-deoxySL on axons can be reversed with S1P.
© 2020 International Society for Neurochemistry.
-
IHC
-
Mus musculus (House mouse)
-
Neuroscience
In Nat Commun on 13 June 2023 by Xie, T., Liu, P., et al.
Fig.6.B

-
WB
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 1