ABSTRACT Endosomal retrieval and recycling of integral cargo proteins is essential for cell, tissue and organism-level development and homeostasis and is orchestrated through a specialised retrieval sub-domain on the endosomal vacuole. However, although sub-domain dysfunction is associated with human disease our appreciation of the molecular details and functional components of the retrieval sub-domain(s) remains poorly described. Here, using comparative proximity proteomics of critical retrieval sub-domain components Retromer and Retriever, their cargo adaptors, and a component of the opposing ESCRT-degradative sub-domain, we provide a data-rich resource that identifies new molecular details of retrieval sub-domain composition and organization, including an unrecognised complexity in the interface of Retromer with RAB GTPases. Combining X-ray crystallography and in silico predictions with extensive biochemical and cellular analysis, we dissect the direct association of Retromer with RAB10 regulators DENND4A, DENND4C, TBC1D1, and TBC1D4, and the RAB35 regulator TBC1D13. Overall, we conclude that the Retromer retrieval sub-domain constitutes a major hub for the regulated switching of selected RAB GTPases and propose that this constitutes a major component of the role of Retromer in neuroprotection.
Product Citations: 17
Mapping the endosomal proximity proteome reveals Retromer as a hub for RAB GTPase regulation
Preprint on BioRxiv : the Preprint Server for Biology on 22 November 2024 by Antón-Plágaro, C., Chen, K., et al.
-
Homo sapiens (Human)
-
Cell Biology
SNX8 enables lysosome reformation and reverses lysosomal storage disorder.
In Nature Communications on 22 March 2024 by Li, X., Xiang, C., et al.
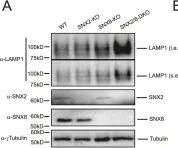
Lysosomal Storage Disorders (LSDs), which share common phenotypes, including enlarged lysosomes and defective lysosomal storage, are caused by mutations in lysosome-related genes. Although gene therapies and enzyme replacement therapies have been explored, there are currently no effective routine therapies against LSDs. During lysosome reformation, which occurs when the functional lysosome pool is reduced, lysosomal lipids and proteins are recycled to restore lysosome functions. Here we report that the sorting nexin protein SNX8 promotes lysosome tubulation, a process that is required for lysosome reformation, and that loss of SNX8 leads to phenotypes characteristic of LSDs in human cells. SNX8 overexpression rescued features of LSDs in cells, and AAV-based delivery of SNX8 to the brain rescued LSD phenotypes in mice. Importantly, by screening a natural compound library, we identified three small molecules that enhanced SNX8-lysosome binding and reversed LSD phenotypes in human cells and in mice. Altogether, our results provide a potential solution for the treatment of LSDs.
© 2024. The Author(s).
-
WB
-
Cell Biology
In Life Science Alliance on 1 March 2023 by Da Graca, J., Charles, J., et al.
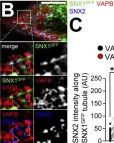
Nutrient deprivation ("starvation") is a major catabolic stress faced by mammalian cells in both pathological and physiological situations. Starvation induces autophagosome biogenesis in the immediate vicinity of ER and leads to lysosome spatial repositioning, but little is known about the consequences of nutritional stress on endosomes. Here, we report that starvation induces tethering of endosomal tubules to ER subregions, fostering autophagosome assembly. We show that this endosomal membrane generation is regulated by sorting nexin 1 (SNX1) protein and is important for the autophagic response. These newly formed SNX1 endosomal tubules establish connections with ER subdomains engaged in early autophagic machinery mobilization. Such endosome-ER transient tethers are regulated by a local dialog between SNX2, an endosomal partner of SNX1, and VAPB, an ER protein associated with autophagy initiation stage regulation. We propose that in a very early response to starvation, SNX1 and SNX2 cooperation induces and regulates endosomal membrane tubulation towards VAPB-positive ER subdomains involved in autophagosome biogenesis, highlighting the contribution of early endosomes in the cellular response to nutritional stress.
© 2022 Da Graça et al.
-
ICC-IF
-
Homo sapiens (Human)
-
Cell Biology
PI4P and BLOC-1 remodel endosomal membranes into tubules.
In The Journal of Cell Biology on 7 November 2022 by Jani, R. A., Di Cicco, A., et al.
Intracellular trafficking is mediated by transport carriers that originate by membrane remodeling from donor organelles. Tubular carriers contribute to the flux of membrane lipids and proteins to acceptor organelles, but how lipids and proteins impose a tubular geometry on the carriers is incompletely understood. Using imaging approaches on cells and in vitro membrane systems, we show that phosphatidylinositol-4-phosphate (PI4P) and biogenesis of lysosome-related organelles complex 1 (BLOC-1) govern the formation, stability, and functions of recycling endosomal tubules. In vitro, BLOC-1 binds and tubulates negatively charged membranes, including those containing PI4P. In cells, endosomal PI4P production by type II PI4-kinases is needed to form and stabilize BLOC-1-dependent recycling endosomal tubules. Decreased PI4KIIs expression impairs the recycling of endosomal cargoes and the life cycles of intracellular pathogens such as Chlamydia bacteria and influenza virus that exploit the membrane dynamics of recycling endosomes. This study demonstrates how a phospholipid and a protein complex coordinate the remodeling of cellular membranes into functional tubules.
© 2022 Jani et al.
-
Cell Biology
Sorting nexin 6 interacts with Cullin3 and regulates programmed death ligand 1 expression.
In FEBS Letters on 1 October 2021 by Ghosh, C., Xing, Y., et al.
Programmed death ligand 1 (PD-L1) is critical for the ability of cancer cells to evade attacks by the host immune system. However, the molecular mechanisms controlling PD-L1 expression have not been fully understood. Here, we demonstrate that sorting nexin 6 (SNX6) is a novel regulator of PD-L1 expression. Knockdown of SNX6 in cancer cells significantly decreases PD-L1 protein levels. In contrast, loss of SNX6 does not reduce PD-L1 mRNA levels. Instead, SNX6 interacts with Cullin3, an E3 ubiquitin ligase responsible for PD-L1 ubiquitination and subsequent degradation. By binding with Cullin3, SNX6 decreases the interaction between the adaptor protein speckle-type POZ protein and Cullin3, which in turn downregulates Cullin3-mediated PD-L1 ubiquitination. This research reveals a novel molecular nexus in modulating PD-L1.
© 2021 Federation of European Biochemical Societies.
-
Homo sapiens (Human)
In Nat Commun on 22 March 2024 by Li, X., Xiang, C., et al.
Fig.3.A

-
WB
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 4
In Life Sci Alliance on 1 March 2023 by Da Graca, J., Charles, J., et al.
Fig.6.B

-
ICC-IF
-
Homo sapiens (Human)
Collected and cropped from Life Sci Alliance by CiteAb, provided under a CC-BY license
Image 1 of 4
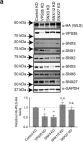
In Nat Commun on 13 September 2018 by McGough, I. J., de Groot, R. E. A., et al.
Fig.3.A

-
WB
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 4
In EMBO J on 17 January 2018 by Jimenez-Orgaz, A., Kvainickas, A., et al.
Fig.3.B

-
WB
-
Homo sapiens (Human)
Collected and cropped from EMBO J by CiteAb, provided under a CC-BY license
Image 1 of 4