Background Rhabdomyosarcoma (RMS) is a highly aggressive pediatric soft tissue sarcoma with limited therapeutic options, particularly for cases resistant to conventional treatments. The SUMOylation pathway, which plays a key role in regulating cell cycle, apoptosis, and transcription, has emerged as a potential therapeutic target in RMS. Elevated levels of SUMO1 and SUMO2/3 conjugates in RMS cell lines, compared to normal human skeletal muscle cells, underscore the association between hyper-SUMOylation and aggressive cancer phenotypes. Understanding these molecular underpinnings is critical for the development of innovative and effective treatments. Methods The investigation encompassed transcriptomic and protein analyses to profile SUMOylation pathway components across alveolar and embryonal RMS subtypes, aiming to identify heterogeneity that could guide personalized therapy approaches. TAK-981’s interactions with chemotherapeutic agents, were evaluated for synergistic effects. Additionally, its impact on radiosensitivity and key signaling pathways, such as AKT and ERK phosphorylation, was assessed to elucidate its mechanism of action. Results Transcriptomic and proteomic analyses revealed distinct expression profiles of SUMOylation pathway components across RMS subtypes, highlighting heterogeneity that could guide personalized therapeutic strategies. Notably, SAE1 protein was overexpressed in RMS tissues and cells, positioning it as a potential biomarker for this cancer. Its activity was effectively counteracted by TAK-981, a SUMO inhibitor that demonstrated significant therapeutic potential by suppressing RMS cell proliferation and migration and enhancing the cytotoxic effects of chemotherapeutic agents, actinomycin D and doxorubicin. However, TAK-981 did not increase radiosensitivity, suggesting its selective action through chemical inhibition mechanisms. Mechanistically, TAK-981 reduced phosphorylation of key signaling proteins, including AKT and ERK, critical for RMS cell survival. Conclusion The findings of this study establish TAK-981 as a promising therapeutic agent for RMS. The results also provide foundational insights into the role of SUMOylation associated to the new biomarker SAE1 in RMS and its subtypes, paving the way for the development of personalized treatment strategies that leverage SUMO pathway inhibition.
Product Citations: 25
Preprint on BioRxiv : the Preprint Server for Biology on 19 January 2025 by Codenotti, S., Lauschke, V. M., et al.
-
Cancer Research
In Cancers on 20 February 2024 by Codenotti, S., Sandrini, L., et al.
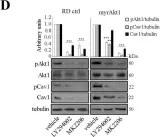
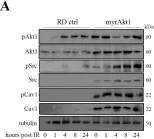
Identifying the molecular mechanisms underlying radioresistance is a priority for the treatment of RMS, a myogenic tumor accounting for approximately 50% of all pediatric soft tissue sarcomas. We found that irradiation (IR) transiently increased phosphorylation of Akt1, Src, and Cav1 in human RD and RH30 lines. Synthetic inhibition of Akt1 and Src phosphorylation increased ROS levels in all RMS lines, promoting cellular radiosensitization. Accordingly, the elevated activation of the Akt1/Src/Cav1 pathway, as detected in two RD lines characterized by overexpression of a myristoylated Akt1 form (myrAkt1) or Cav1 (RDCav1), was correlated with reduced levels of ROS, higher expression of catalase, and increased radioresistance. We found that treatment with cholesterol-lowering drugs such as lovastatin and simvastatin promoted cell apoptosis in all RMS lines by reducing Akt1 and Cav1 levels and increasing intracellular ROS levels. Combining statins with IR significantly increased DNA damage and cell apoptosis as assessed by γ histone 2AX (γH2AX) staining and FACS analysis. Furthermore, in combination with the chemotherapeutic agent actinomycin D, statins were effective in reducing cell survival through increased apoptosis. Taken together, our findings suggest that the molecularly linked signature formed by Akt1, Src, Cav1, and catalase may represent a prognostic determinant for identifying subgroups of RMS patients with higher probability of recurrence after radiotherapy. Furthermore, statin-induced oxidative stress could represent a treatment option to improve the success of radiotherapy.
-
WB
-
Homo sapiens (Human)
-
Cancer Research
A Role of Caveolae in Trabecular Meshwork Mechanosensing and Contractile Tone.
In Frontiers in Cell and Developmental Biology on 5 April 2022 by De Ieso, M. L., Kuhn, M., et al.
Polymorphisms in the CAV1/2 gene loci impart increased risk for primary open-angle glaucoma (POAG). CAV1 encodes caveolin-1 (Cav1), which is required for biosynthesis of plasma membrane invaginations called caveolae. Cav1 knockout mice exhibit elevated intraocular pressure (IOP) and decreased outflow facility, but the mechanistic role of Cav1 in IOP homeostasis is unknown. We hypothesized that caveolae sequester/inhibit RhoA, to regulate trabecular meshwork (TM) mechanosensing and contractile tone. Using phosphorylated myosin light chain (pMLC) as a surrogate indicator for Rho/ROCK activity and contractile tone, we found that pMLC was elevated in Cav1-deficient TM cells compared to control (131 ± 10%, n = 10, p = 0.016). Elevation of pMLC levels following Cav1 knockdown occurred in cells on a soft surface (137 ± 7%, n = 24, p < 0.0001), but not on a hard surface (122 ± 17%, n = 12, p = 0.22). In Cav1-deficient TM cells where pMLC was elevated, Rho activity was also increased (123 ± 7%, n = 6, p = 0.017), suggesting activation of the Rho/ROCK pathway. Cyclic stretch reduced pMLC/MLC levels in TM cells (69 ± 7% n = 9, p = 0.002) and in Cav1-deficient TM cells, although not significantly (77 ± 11% n = 10, p = 0.059). Treatment with the Cav1 scaffolding domain mimetic, cavtratin (1 μM) caused a reduction in pMLC (70 ± 5% n = 7, p = 0.001), as did treatment with the scaffolding domain mutant cavnoxin (1 μM) (82 ± 7% n = 7, p = 0.04). Data suggest that caveolae differentially regulate RhoA signaling, and that caveolae participate in TM mechanotransduction. Cav1 regulation of these key TM functions provide evidence for underlying mechanisms linking polymorphisms in the Cav1/2 gene loci with increased POAG risk.
Copyright © 2022 De Ieso, Kuhn, Bernatchez, Elliott and Stamer.
-
WB
In Cancer Letters on 1 May 2021 by Codenotti, S., Marampon, F., et al.
The aim of this work was to investigate whether Caveolin-1 (Cav-1), a membrane scaffolding protein widely implicated in cancer, may play a role in radiation response in rhabdomyosarcoma (RMS), a pediatric soft tissue tumor. For this purpose, we employed human RD cells in which Cav-1 expression was stably increased via gene transfection. After radiation treatment, we observed that Cav-1 limited cell cycle arrest in the G2/M phase and enhanced resistance to cell senescence and apoptosis via reduction of p21Cip1/Waf1, p16INK4a and Caspase-3 cleavage. After radiotherapy, Cav-1-mediated cell radioresistance was characterized by low accumulation of H2AX foci, as confirmed by Comet assay, marked neutralization of reactive oxygen species (ROS) and enhanced DNA repair via activation of ATM, Ku70/80 complex and DNA-PK. We found that Cav-1-overexpressing RD cells, already under basal conditions, had higher glutathione (GSH) content and greater catalase expression, which conferred protection against acute treatment with hydrogen peroxide. Furthermore, pre-treatment of Cav-1-overexpressing cells with PP2 or LY294002 compounds restored the sensitivity to radiation treatment, indicating a role for Src-kinases and Akt pathways in Cav-1-mediated radioresistance. These findings were confirmed using radioresistant RD and RH30 lines generated by hypofractionated radiotherapy protocol, which showed marked increase of Cav-1, catalase and Akt, and sensitivity to PP2 and LY294002 treatment. In conclusion, these data suggest that concerted activity of Cav-1 and catalase, in cooperation with activation of Src-kinase and Akt pathways, may represent a network of vital mechanisms that allow irradiated RMS cells to evade cell death induced by oxidative stress and DNA damage.
Copyright © 2021 Elsevier B.V. All rights reserved.
-
Cancer Research
-
Genetics
In FEBS Letters on 1 February 2021 by Buwa, N., Kannan, N., et al.
Integrin-mediated adhesion regulates cellular responses to changes in the mechanical and biochemical properties of the extracellular matrix. Cell-matrix adhesion regulates caveolar endocytosis, dependent on caveolin 1 (Cav1) Tyr14 phosphorylation (pY14Cav1), to control anchorage-dependent signaling. We find that cell-matrix adhesion regulates pY14Cav1 levels in mouse fibroblasts. Biochemical fractionation reveals endogenous pY14Cav1 to be present in caveolae and focal adhesions (FA). Adhesion does not affect caveolar pY14Cav1, supporting its regulation at FA, in which PF-228-mediated inhibition of focal adhesion kinase (FAK) disrupts. Cell adhesion on 2D polyacrylamide matrices of increasing stiffness stimulates Cav1 phosphorylation, which is comparable to the phosphorylation of FAK. Inhibition of FAK across varying stiffnesses shows it regulates pY14Cav1 more prominently at higher stiffness. Taken together, these studies reveal the presence of FAK-pY14Cav1 crosstalk at FA, which is regulated by cell-matrix adhesion.
© 2020 Federation of European Biochemical Societies.
-
WB
-
Mus musculus (House mouse)
In Cancers (Basel) on 20 February 2024 by Codenotti, S., Sandrini, L., et al.
Fig.1.C

-
WB
-
Homo sapiens (Human)
Collected and cropped from Cancers (Basel) by CiteAb, provided under a CC-BY license
Image 1 of 6
In Cancers (Basel) on 20 February 2024 by Codenotti, S., Sandrini, L., et al.
Fig.2.D

-
WB
-
Homo sapiens (Human)
Collected and cropped from Cancers (Basel) by CiteAb, provided under a CC-BY license
Image 1 of 6
In Cancers (Basel) on 20 February 2024 by Codenotti, S., Sandrini, L., et al.
Fig.2.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Cancers (Basel) by CiteAb, provided under a CC-BY license
Image 1 of 6
In Cancers (Basel) on 20 February 2024 by Codenotti, S., Sandrini, L., et al.
Fig.3.B

-
WB
-
Homo sapiens (Human)
Collected and cropped from Cancers (Basel) by CiteAb, provided under a CC-BY license
Image 1 of 6
In Cancers (Basel) on 20 February 2024 by Codenotti, S., Sandrini, L., et al.
Fig.3.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Cancers (Basel) by CiteAb, provided under a CC-BY license
Image 1 of 6
In Cancers (Basel) on 20 February 2024 by Codenotti, S., Sandrini, L., et al.
Fig.4.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Cancers (Basel) by CiteAb, provided under a CC-BY license
Image 1 of 6