Lysosomes are membrane-bound organelles critical for maintaining cellular homeostasis. Delivery of biosynthetic lysosomal proteins to lysosomes is crucial to orchestrate proper lysosomal function. However, it remains unknown how the delivery of biosynthetic lysosomal proteins to lysosomes is ensured in neurons, which are highly polarized cells. Here, we developed Protein Origin, Trafficking And Targeting to Organelle Mapping (POTATOMap), by combining trafficking synchronization and proximity-labelling based proteomics, to unravel the trafficking routes and interactome of the biosynthetic lysosomal membrane protein LAMP1 at specified time points. This approach, combined with advanced microscopy, enables us to identify the neuronal domain-specific trafficking machineries of biosynthetic LAMP1. We reveal a role in replenishing axonal lysosomes, in delivery of newly synthesized axonal synaptic proteins, and interactions with RNA granules to facilitate hitchhiking in the axon. POTATOMap offers a robust approach to map out dynamic biosynthetic protein trafficking and interactome from their origin to destination.
© 2025. The Author(s).
Product Citations: 12
In Nature Communications on 30 December 2024 by Li, C. H., Kersten, N., et al.
-
Cell Biology
-
Neuroscience
Spatiotemporal proteomics reveals the biosynthetic lysosomal membrane protein interactome in neurons
Preprint on BioRxiv : the Preprint Server for Biology on 16 May 2024 by Li, C. H., Kersten, N., et al.
Lysosomes are membrane-bound organelles critical for maintaining cellular homeostasis. Delivery of biosynthetic lysosomal proteins to lysosomes is crucial to orchestrate proper lysosomal function. However, it remains unknown how the delivery of biosynthetic lysosomal proteins to lysosomes is ensured in neurons, which are highly polarized cells. Here, we developed Protein Origin, Trafficking And Targeting to Organelle Mapping (POTATOMap), by combining trafficking synchronization and proximity-labelling based proteomics, to unravel the trafficking routes and interactome of the biosynthetic lysosomal membrane protein LAMP1 at specified time points. This approach, combined with advanced microscopy, enabled us to identify the neuronal domain-specific trafficking machineries of biosynthetic LAMP1. We revealed a role in replenishing axonal lysosomes, in delivery of newly synthesized axonal synaptic proteins, and interactions with RNA granules to facilitate hitchhiking in the axon. POTATOMap offers a robust approach to map out dynamic biosynthetic protein trafficking and interactome from their origin to destination.
-
Cell Biology
-
Neuroscience
Syntaxin-5's flexibility in SNARE pairing supports Golgi functions.
In Traffic (Copenhagen, Denmark) on 1 August 2023 by D'Souza, Z., Pokrovskaya, I., et al.
Deficiency in the conserved oligomeric Golgi (COG) complex that orchestrates SNARE-mediated tethering/fusion of vesicles that recycle the Golgi's glycosylation machinery results in severe glycosylation defects. Although two major Golgi v-SNAREs, GS28/GOSR1, and GS15/BET1L, are depleted in COG-deficient cells, the complete knockout of GS28 and GS15 only modestly affects Golgi glycosylation, indicating the existence of an adaptation mechanism in Golgi SNARE. Indeed, quantitative mass-spectrometry analysis of STX5-interacting proteins revealed two novel Golgi SNARE complexes-STX5/SNAP29/VAMP7 and STX5/VTI1B/STX8/YKT6. These complexes are present in wild-type cells, but their usage is significantly increased in both GS28- and COG-deficient cells. Upon GS28 deletion, SNAP29 increased its Golgi residency in a STX5-dependent manner. While STX5 depletion and Retro2-induced diversion from the Golgi severely affect protein glycosylation, GS28/SNAP29 and GS28/VTI1B double knockouts alter glycosylation similarly to GS28 KO, indicating that a single STX5-based SNARE complex is sufficient to support Golgi glycosylation. Importantly, co-depletion of three Golgi SNARE complexes in GS28/SNAP29/VTI1B TKO cells resulted in severe glycosylation defects and a reduced capacity for glycosylation enzyme retention at the Golgi. This study demonstrates the remarkable plasticity in SXT5-mediated membrane trafficking, uncovering a novel adaptive response to the failure of canonical intra-Golgi vesicle tethering/fusion machinery.
© 2023 The Authors. Traffic published by John Wiley & Sons Ltd.
-
Cell Biology
S-Palmitoylation of Tyrosinase at Cysteine500 Regulates Melanogenesis.
In The Journal of Investigative Dermatology on 1 February 2023 by Niki, Y., Adachi, N., et al.
Palmitoylation is a lipid modification involving the attachment of palmitic acid to a cysteine residue, thereby affecting protein function. We investigated the effect of palmitoylation of tyrosinase, the rate-limiting enzyme in melanin synthesis, using a human three-dimensional skin model system and melanocyte culture. The palmitoylation inhibitor, 2-bromopalmitate, increased melanin content and tyrosinase protein levels in melanogenic cells by suppressing tyrosinase degradation. The palmitoylation site was Cysteine500 in the C-terminal cytoplasmic tail of tyrosinase. The nonpalmitoylatable mutant, tyrosinase (C500A), was slowly degraded and less ubiquitinated than wild-type tyrosinase. Screening for the Asp-His-His-Cys (DHHC) family of proteins for tyrosinase palmitoylation suggested that DHHC2, 3, 7, and 15 are involved in tyrosinase palmitoylation. Knockdown of DHHC2, 3, or 15 increased tyrosinase protein levels and melanin content. Determination of their subcellular localization in primary melanocytes revealed that DHHC2, 3, and 15 were localized in the endoplasmic reticulum, Golgi apparatus, and/or melanosomes, whereas only DHHC2 was localized in the melanosomes. Immunoprecipitation showed that DHHC2 and DHHC3 predominantly bind to mature and immature tyrosinase, respectively. Taken together, tyrosinase palmitoylation at Cysteine500 by DHHC2, 3, and/or 15, especially DHHC2 in trans-Golgi apparatus and melanosomes and DHHC3 in the endoplasmic reticulum and cis-Golgi apparatus, regulate melanogenesis by modulating tyrosinase protein levels.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
STX5’s flexibility in SNARE pairing supports Golgi functions
Preprint on BioRxiv : the Preprint Server for Biology on 25 May 2022 by D’Souza, Z., Pokrovskaya, I., et al.
The intracellular transport system is an evolutionally conserved, essential, and highly regulated network of organelles and transport vesicles that traffic protein and lipid cargoes within the cell. The events of vesicle formation, budding and fusion are orchestrated by the trafficking machinery – an elaborate set of proteins including small GTPases, vesicular coats, tethers, and SNAREs. The Golgi - the central organelle in this transport network, receives, modifies and sorts secretory and endocytic cargo. Glycosylation is one of the major modifications that occur within the Golgi, which houses enzymes and other components of glycosylation machinery. According to the current Golgi maturation model, Golgi resident proteins are constantly recycled from the late (trans) Golgi compartments to the early compartment (cis) by the evolutionary conserved vesicular trafficking machinery. The key modulator of vesicular trafficking and glycosylation at the Golgi is the Conserved Oligomeric Golgi (COG) complex – its interaction vesicular trafficking machinery particularly Golgi SNAREs (STX5, GS28 (GOSR1), GS15 (BET1L) and YKT6) that drive fusion of incoming vesicles. Since the COG complex functions upstream of SNARE-mediated vesicle fusion, we hypothesize that depletion of Golgi v-SNAREs would mirror defects observed in COG deficient cells. To test this, we created single and double knockouts (KO) of GS28 and GS15 in HEK293T cells and analyzed resulting mutants using a comprehensive set of biochemical, mass-spectrometry (MS) and microscopy approaches. Deletion of GS28 significantly affected GS15, but not the other two partners, STX5 and YKT6. Surprisingly, our analysis revealed that COG dysfunction is more deleterious for Golgi function than disrupting the canonical Golgi SNARE complex. Quantitative MS analysis of STX5-interacting SNAREs revealed unexpected flexibility of Golgi SNARE pairing in mammalian cells. We uncovered two novel non-canonical Golgi SNARE complexes – STX5/VTI1B/GS15/YKT6 and STX5/SNAP29/VAMP7 which were upregulated in GS28 KO cells. Analysis of cells co-depleted for GS28/SNAP29 or GS28/VTI1B SNAREs revealed escalated defects in Golgi glycosylation, indicating that upregulation of these complexes functionally substitutes deleted GS28. Our data points to the remarkable plasticity in the intra-Golgi membrane fusion machinery which is controlled by the COG complex.
-
Cell Biology
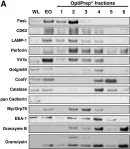
In BMC Immunol on 29 July 2009 by Schmidt, H., Gelhaus, C., et al.
Fig.2.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from BMC Immunol by CiteAb, provided under a CC-BY license
Image 1 of 1