Given that plasminogen activator inhibitor 1 (PAI-1) plays an important role in human pathobiology and epigallocatechin-3-gallate (EGCG) exerts vasculoprotective actions, we investigated the role(s) of PAI-1 and the protective effect of EGCG in the mechanism of AAA formation, with a focus on inflammation, oxidative stress, proteolysis, and apoptosis in vivo and in vitro. Nine-week-old wild-type mice (PAI-1+/+) and PAI-1 deficiency mice (PAI-1-/-) randomly assigned to the sham operation (0.9% saline) and AAA induction (calcium chloride) and subjected to biological and morphological analysis after four weeks. On operative day 28, the AAA lesions had decreased levels of PAI-1 mRNA and protein. As compared with AAA-PAI-1+/+ mice, PAI-1 deficiency aggravated AAA formation accompanied by plasma TNF-α and IL-1β elevations. PAI-1-/- resulted in harmful changes in the levels of gp91phox, cleaved-caspase 8, TGF-β, p-Smad2/3, collagen I/III, gp91phox, ICAM-1, VCAM-1 mRNAs and/or protein in the AAA lesions as well as oxidative stress production and macrophage infiltration. PAI-1-/- also increased elastin degradation and collagen accumulation associated with the reduction of proteolytic MMP-2/-9 expressions and activities. While EGCG reversed the above changes and upregulated PAI-1 expression. In vitro, PAI-1 inhibition (silencing and pharmacological inhibitor) and overexpression, respectively, increased and lowered oxidative stress-induced VSMCs apoptosis and investigated extracellular protein turnover-related protein changes. These results suggested that the protective role of PAI-1 and EGCG in AAA formation is based on their ability to inhibit inflammation, oxidative stress, and apoptosis. Moreover, EGCG-mediated PAI-1 induction might provide a potential pharmacological treatment for AAA.
© 2025 The Author(s). The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Product Citations: 50
In The FASEB Journal on 15 May 2025 by Zhao, M., Hu, L., et al.
In Scientific Reports on 9 January 2025 by Čináková, A., Vavrincova-Yaghi, D., et al.
Oxidative stress and apoptosis are highly engaged in development of diabetic nephropathy (DN). In monotherapy, dapagliflozin and pioglitazone positively modulate target organ damage even independently of their hypoglycaemic effect. This study evaluated whether a simultaneous PPARγ activation and SGLT cotransporter inhibition offer superior protection against DN-related oxidative and apoptotic processes in a T1DM rat model. Diabetes was induced in Wistar rats using streptozotocin (55 mg/kg, i.p.). The rats received daily chow containing dapagliflozin (10 mg/kg), pioglitazone (12 mg/kg) or their combination. Six weeks after STZ administration, histological and molecular analyses were performed in excised kidneys. STZ-induced DN was demonstrated by the propagation of apoptotic (Bax, p53, Casp3) and oxidative reactions (Gp91phox, MnSOD) and disrupted nitric oxide signalling (eNOS, Hsp90, Cav1). Kidney damage molecule expression (Kim1, Nphs1) revealed a deceleration of kidney damage by pioglitazone and dapagliflozine monotherapies. The monotherapy also reduced apoptosis, oxidative stress, and partially restored NO signalling. The combined therapy ameliorated glomerulosclerosis but in other measured parameters, it reached the effect of the monotherapies except for Hsp90 expression modulation. Both dapagliflozin and pioglitazone exert protective character in kidneys when used in monotherapy. The combined therapy does not exhibit an expected additive effect within modulating oxidative stress, NO signalling or apoptosis.
© 2025. The Author(s).
-
WB
-
Rattus norvegicus (Rat)
In Antioxidants (Basel, Switzerland) on 7 January 2025 by Kuntic, M., Kuntić, I., et al.
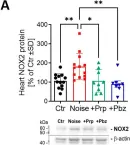
Noise pollution is a known health risk factor and evidence for cardiovascular diseases associated with traffic noise is growing. At least 20% of the European Union's population lives in noise-polluted areas with exposure levels exceeding the recommended limits of the World Health Organization, which is considered unhealthy by the European Environment Agency. This results in the annual loss of 1.6 million healthy life years. Here, we investigated the protective effects of cardiovascular drug interventions against aircraft noise-mediated cardiovascular complications such as elevated oxidative stress or endothelial dysfunction. Using our established mouse exposure model, we applied mean sound pressure levels of 72 dB(A) for 4 d. C57BL/6 mice were treated with the beta-blocker propranolol (15 mg/kg/d s.c. for 5 d) or the alpha-blocker phenoxybenzamine (1.5 mg/kg/d s.c. for 5 d) and noise-exposed for the last 4 d of the drug administration. Short-term noise exposure caused hypertension (measured by tail-cuff blood pressure monitoring) and impaired endothelial function (measured by isometric tension recording in the aorta and video microscopy in cerebral arterioles in response to acetylcholine). Noise also increased markers of oxidative stress and inflammation. Treatment of mice with propranolol and phenoxybenzamine prevented endothelial and microvascular dysfunction, which was supported by a decrease in markers of inflammation and oxidative stress in heart tissue and the brain. Amelioration of noise-induced hypertension (systolic blood pressure) was not observed, whereas pulse pressure was lowered by trend. This study provides a novel perspective mitigating the adverse effects of noise pollution, especially in vulnerable groups with medication, a rationale for further pharmacological human studies.
-
WB
-
Cardiovascular biology
In Journal of Hypertension on 1 November 2024 by Lin, Z., Zhao, M., et al.
Abdominal aortic aneurysm (AAA) is an aneurysm-like dilated and highly fatal cardiovascular disease. CD8 + T cells have been shown to be critical for vascular pathological processes, but the contribution of these lymphocytes to vascular diseases remains elusive.
Eight-week-old male wildtype (CD8 +/+ ) and Cd8a knockout (CD8 -/- ) mice were used in a calcium chloride 2 (CaCl 2 )-induced experimental AAA model. At 6 weeks after surgery, CD8 + T-cell deletion prevented the formation of AAA, accompanied by reductions of the levels of inflammatory (interferon-γ [IFN-γ], interleukin-1β, monocyte chemoattractant protein-1, intracellular adhesion molecule-1, vascular cell adhesion molecule-1, NOD-like receptor protein 3, caspase-1), oxidative stress [NADPH oxidase and gp91 phox ], and proteolysis (cathepsin S, cathepsin K, matrix metalloproteinase-2 [MMP-2] and MMP-9) proteins and/or genes in plasma and/or AAA tissues. Immunoreactivities of MMP-2 and MMP-9 were observed in macrophages. An injection of IFN-γ and adoptive transfer of CD8 + T cells of IFN-γ +/+ mice diminished CD8 -/- -mediated vasculoprotective actions in the AAA mice. In vitro, IFN-γ enhanced MMP-2 and MMP-9 gelatinolytic activities in macrophage and/or vascular smooth muscle cells.
The vasculoprotective effects of CD8 + T-cell deletion in a mouse CaCl 2 -induced AAA model were likely attributable to, at least in part, the attenuation of IFN-γ-dependent inflammation action, oxidative stress production, and proteolysis, suggesting a novel therapeutic target for AAA formation by regulating CD8 + T-cell-derived IFN-γ secretion.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
-
Cardiovascular biology
-
Immunology and Microbiology
In Journal of Lipid Research on 1 December 2022 by Yuan, X., Bhat, O. M., et al.
The NOD-like receptor pyrin domain 3 (NLRP3) inflammasome is activated during atherogenesis, but how this occurs is unclear. Here, we explored the mechanisms activating and regulating NLRP3 inflammasomes via the acid sphingomyelinase (ASM)-ceramide signaling pathway. As a neointima formation model, partial left carotid ligations were performed on endothelial cell (EC)-specific ASM transgene mice (Smpd1trg/ECcre) and their control littermates (Smpd1trg/WT and WT/WT) fed on the Western diet (WD). We found neointima formation remarkably increased in Smpd1trg/ECcre mice over their control littermates. Next, we observed enhanced colocalization of NLRP3 versus adaptor protein ASC (the adaptor molecule apoptosis-associated speck-like protein containing a CARD) or caspase-1 in the carotid ECs of WD-treated Smpd1trg/ECcre mice but not in their control littermates. In addition, we used membrane raft (MR) marker flotillin-1 and found more aggregation of ASM and ceramide in the intima of Smpd1trg/ECcre mice than their control littermates. Moreover, we demonstrated by in situ dihydroethidium staining, carotid intimal superoxide levels were much higher in WD-treated Smpd1trg/ECcre mice than in their control littermates. Using ECs from Smpd1trg/ECcre and WT/WT mice, we showed ASM overexpression markedly enhanced 7-ketocholesterol (7-Ket)-induced increases in NLRP3 inflammasome formation, accompanied by enhanced caspase-1 activity and elevated interleukin-1β levels. These 7-Ket-induced increases were significantly attenuated by ASM inhibitor amitriptyline. Furthermore, we determined that increased MR clustering with NADPH oxidase subunits to produce superoxide contributes to 7-Ket-induced NLRP3 inflammasome activation via a thioredoxin-interacting protein-mediated controlling mechanism. We conclude that ceramide from ASM plays a critical role in NLRP3 inflammasome activation during hypercholesterolemia via MR redox signaling platforms to produce superoxide, which leads to TXNIP dissociation.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
-
Mus musculus (House mouse)
In Antioxidants (Basel) on 7 January 2025 by Kuntic, M., Kuntić, I., et al.
Fig.5.A

-
WB
-
Collected and cropped from Antioxidants (Basel) by CiteAb, provided under a CC-BY license
Image 1 of 6
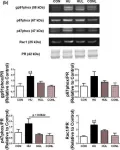
In Arterioscler Thromb Vasc Biol on 1 February 2021 by Harrison, C. B., Trevelin, S. C., et al.
Fig.4.C

-
WB
-
Collected and cropped from Arterioscler Thromb Vasc Biol by CiteAb, provided under a CC-BY license
Image 1 of 6
In Physiol Rep on 1 January 2021 by Hord, J. M., Garcia, M. M., et al.
Fig.3.B

-
WB
-
Collected and cropped from Physiol Rep by CiteAb, provided under a CC-BY license
Image 1 of 6
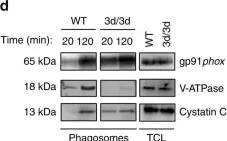
In Nat Commun on 21 November 2017 by Maschalidi, S., Nunes-Hasler, P., et al.
Fig.2.D

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 6
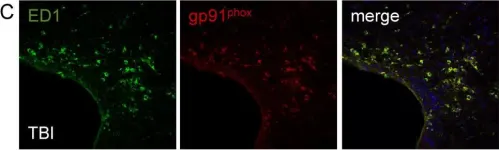
In J Neuroinflammation on 28 February 2012 by Byrnes, K. R., Loane, D. J., et al.
Fig.1.C

-
IHC-IF
-
Mus musculus (House mouse)
Collected and cropped from J Neuroinflammation by CiteAb, provided under a CC-BY license
Image 1 of 6
In J Neuroinflammation on 28 February 2012 by Byrnes, K. R., Loane, D. J., et al.
Fig.5.A

-
IHC-IF
-
Mus musculus (House mouse)
Collected and cropped from J Neuroinflammation by CiteAb, provided under a CC-BY license
Image 1 of 6