In schizophrenia (SCZ), neurons in the brain tend to undergo gross morphological changes, but the related molecular mechanism remains largely elusive. Using Kif3b+/- mice as a model with SCZ-like behaviors, we found that a high-betaine diet can significantly alleviate schizophrenic traits related to neuronal morphogenesis and behaviors. According to a deficiency in the transport of collapsin response mediator protein 2 (CRMP2) by the KIF3 motor, we identified a significant reduction in lamellipodial dynamics in developing Kif3b+/- neurons as a cause of neurite hyperbranching. Betaine administration significantly decreases CRMP2 carbonylation, which enhances the F-actin bundling needed for proper lamellipodial dynamics and microtubule exclusion and may thus functionally compensate for KIF3 deficiency. Because the KIF3 expression levels tend to be downregulated in the human prefrontal cortex of the postmortem brains of SCZ patients, this mechanism may partly participate in human SCZ pathogenesis, which we hypothesize could be alleviated by betaine administration.Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Product Citations: 16
In Cell Reports on 13 April 2021 by Yoshihara, S., Jiang, X., et al.
In Cancers on 1 April 2021 by Shi, P., Hoang-Minh, L. B., et al.
Histone deacetylase 6 (HDAC6) is an emerging therapeutic target that is overexpressed in glioblastoma when compared to other HDACs. HDAC6 catalyzes the deacetylation of alpha-tubulin and mediates the disassembly of primary cilia, a process required for cell cycle progression. HDAC6 inhibition disrupts glioma proliferation, but whether this effect is dependent on tumor cell primary cilia is unknown. We found that HDAC6 inhibitors ACY-1215 (1215) and ACY-738 (738) inhibited the proliferation of multiple patient-derived and mouse glioma cells. While both inhibitors triggered rapid increases in acetylated alpha-tubulin (aaTub) in the cytosol and led to increased frequencies of primary cilia, they unexpectedly reduced the levels of aaTub in the cilia. To test whether the antiproliferative effects of HDAC6 inhibitors are dependent on tumor cell cilia, we generated patient-derived glioma lines devoid of cilia through depletion of ciliogenesis genes ARL13B or KIF3A. At low concentrations, 1215 or 738 did not decrease the proliferation of cilia-depleted cells. Moreover, the differentiation of glioma cells that was induced by HDAC6 inhibition did not occur after the inhibition of cilia formation. These data suggest HDAC6 signaling at primary cilia promotes the proliferation of glioma cells by restricting their ability to differentiate. Surprisingly, overexpressing HDAC6 did not reduce cilia length or the frequency of ciliated glioma cells, suggesting other factors are required to control HDAC6-mediated cilia disassembly in glioma cells. Collectively, our findings suggest that HDAC6 promotes the proliferation of glioma cells through primary cilia.
-
Cancer Research
In Science Advances on 1 December 2020 by Iwata, S., Morikawa, M., et al.
Synaptic weight changes among postsynaptic densities within a single dendrite are regulated by the balance between localized protein degradation and synthesis. However, the molecular mechanism via these opposing regulatory processes is still elusive. Here, we showed that the molecular motor KIF17 was locally degraded and synthesized in an N-methyl-d-aspartate receptor (NMDAR)-mediated activity-dependent manner. Accompanied by the degradation of KIF17, its transport was temporarily dampened in dendrites. We also observed that activity-dependent local KIF17 synthesis driven by its 3' untranslated region (3'UTR) occurred at dendritic shafts, and the newly synthesized KIF17 moved along the dendrites. Furthermore, hippocampus-specific deletion of Kif17 3'UTR disrupted KIF17 synthesis induced by fear memory retrieval, leading to impairment in extinction of fear memory. These results indicate that the regulation of the KIF17 transport is driven by the single dendrite-restricted cycle of degradation and synthesis that underlies cognitive flexibility.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution License 4.0 (CC BY).
-
Neuroscience
In The EMBO Journal on 2 January 2020 by Alsabban, A. H., Morikawa, M., et al.
The transport of N-methyl-d-aspartate receptors (NMDARs) is crucial for neuronal plasticity and synapse formation. Here, we show that KIF3B, a member of the kinesin superfamily proteins (KIFs), supports the transport of vesicles simultaneously containing NMDAR subunit 2A (NR2A) and the adenomatous polyposis coli (APC) complex. Kif3b+/- neurons exhibited a reduction in dendritic levels of both NR2A and NR2B due to the impaired transport of NR2A and increased degradation of NR2B. In Kif3b+/- hippocampal slices, electrophysiological NMDAR response was found decreased and synaptic plasticity was disrupted, which corresponded to a common feature of schizophrenia (SCZ). The histological features of Kif3b+/- mouse brain also mimicked SCZ features, and Kif3b+/- mice exhibited behavioral defects in prepulse inhibition (PPI), social interest, and cognitive flexibility. Indeed, a mutation of KIF3B was specifically identified in human SCZ patients, which was revealed to be functionally defective in a rescue experiment. Therefore, we propose that KIF3B transports NR2A/APC complex and that its dysfunction is responsible for SCZ pathogenesis.
© 2019 The Authors.
In Cell Reports on 27 August 2019 by Ichinose, S., Ogawa, T., et al.
The axon initial segment (AIS) is a compartment that serves as a molecular barrier to achieve axon-dendrite differentiation. Distribution of specific proteins during early neuronal development has been proposed to be critical for AIS construction. However, it remains unknown how these proteins are specifically targeted to the proximal axon within this limited time period. Here, we reveal spatiotemporal regulation driven by the microtubule (MT)-based motor KIF3A/B/KAP3 that transports TRIM46, influenced by a specific MARK2 phosphorylation cascade. In the proximal part of the future axon under low MARK2 activity, the KIF3/KAP3 motor recognizes TRIM46 as cargo and transports it to the future AIS. In contrast, in the somatodendritic area under high MARK2 activity, KAP3 phosphorylated at serine 60 by MARK2 cannot bind with TRIM46 and be transported. This spatiotemporal regulation between KIF3/KAP3 and TRIM46 under specific MARK2 activity underlies the specific transport needed for axonal differentiation.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
-
Neuroscience
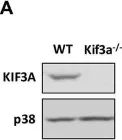
In PLoS One on 28 August 2018 by Ho Wei, L., Arastoo, M., et al.
Fig.1.A

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 4
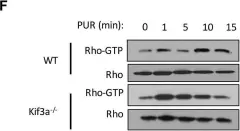
In PLoS One on 28 August 2018 by Ho Wei, L., Arastoo, M., et al.
Fig.1.F

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 4
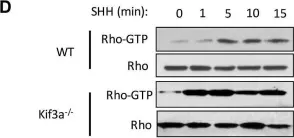
In PLoS One on 28 August 2018 by Ho Wei, L., Arastoo, M., et al.
Fig.1.D

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 4
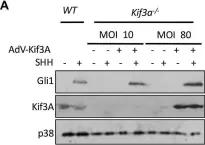
In PLoS One on 28 August 2018 by Ho Wei, L., Arastoo, M., et al.
Fig.2.A

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 4