Breastfeeding protects against breast cancer in some women but not others, however the mechanism remains elusive. Lactation requires intense secretory activity of the endoplasmic reticulum for the production of milk proteins and endoplasmic reticulum- mitochondria contacts for lipid synthesis. We show that in female mice that share the same nuclear genome (BL/6) but differ in mitochondrial genomes (C57 or NZB), lactation engages different transcriptional programs resulting in anti-tumorigenic lactation in BL/6C57 females and pro-tumorigenic lactation in BL/6NZB females. Our data indicate activation of a pro-apoptotic endoplasmic reticulum-stress response during lactation in BL/6C57 females, which is not observed in BL/6NZB females. Single cell sequencing identified a sub-population of cells, uniquely amplified during lactation in BL/6NZB females, that shares the genetic signature of post-partum breast cancer in humans and is characterized by cell cycle markers and loss of p53. We show that pharmacological manipulations of endoplasmic reticulum-stress directly affect this signature. Overall, our data suggest the unexpected differential nature of lactation and its potential impact on the risk of the development of post-partum breast cancer.
© 2025. The Author(s).
Product Citations: 19
In Nature Communications on 11 July 2025 by Chattopadhyay, M., Jenkins, E. C., et al.
-
Cell Biology
Understanding Mitochondrial and Lysosomal Dynamics by Fluorescent Microscopy.
In Methods in Molecular Biology (Clifton, N.J.) on 15 November 2024 by Dionísio, P. A., Sardão, V. A., et al.
Live cell imaging is a robust method to visualize dynamic cellular structures, especially organelles with network-like structures such as mitochondria. In this regard, mitochondrial dynamics, namely mitochondrial fission and fusion, are highly dynamic processes that regulate mitochondrial size and morphology depending on a plethora of cellular cues. Likewise, lysosome size and distribution may hint at their function and state.Here, we describe how to perform live cell confocal imaging using commercially available organelle dyes (MitoTracker, LysoTracker), followed by either 2D or 3D analyses of mitochondrial morphology/network connectivity and lysosomal morphology using the freely available Mitochondria Analyzer plugin for ImageJ/Fiji.
© 2025. The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature.
-
Biochemistry and Molecular biology
-
Cell Biology
Idiosyncratic nature of lactation reveals link to breast cancer risk.
Preprint on Research Square on 28 June 2024 by Germain, D., Chattopadhyay, M., et al.
Abstract Breastfeeding protects against breast cancer in some women but not others, however the mechanism remains elusive. Lactation requires intense secretory activity of the endoplasmic reticulum (ER) for the production of milk proteins and ER- mitochondria contacts for lipid synthesis. We show that in female mice that share the same nuclear genome (BL/6) but differ in mitochondrial genomes (C57 or NZB), the biological processes engaged during lactation are entirely different at the sub-cellular organization and transcriptional levels resulting in anti-tumorigenic lactation in BL/6C57 females and pro-tumorigenic lactation in BL/6NZB females. Single cell sequencing identified a sub-population of cells, uniquely amplified during lactation in BL/6NZB females, which shares the genetic signature that characterizes post-partum breast cancer (PPBC) in humans relative to matched breast cancers in never pregnant women. Our data indicate that differences in ER and mitochondrial-stress responses during lactation between genotypes inadvertently leads to loss of p53 tumor suppressor function in BL/6NZB females allowing the expansion of the PPBC-like sub-population of cells. Overall, our data reveals the unexpected idiosyncratic nature of lactation and its impacts on the risk of the development of PPBC.
-
Mus musculus (House mouse)
-
Cancer Research
NINJ1 mediates plasma membrane rupture by cutting and releasing membrane disks.
In Cell on 25 April 2024 by David, L., Borges, J. P., et al.
The membrane protein NINJ1 mediates plasma membrane rupture in pyroptosis and other lytic cell death pathways. Here, we report the cryo-EM structure of a NINJ1 oligomer segmented from NINJ1 rings. Each NINJ1 subunit comprises amphipathic (⍺1, ⍺2) and transmembrane (TM) helices (⍺3, ⍺4) and forms a chain of subunits, mainly by the TM helices and ⍺1. ⍺3 and ⍺4 are kinked, and the Gly residues are important for function. The NINJ1 oligomer possesses a concave hydrophobic side that should face the membrane and a convex hydrophilic side formed by ⍺1 and ⍺2, presumably upon activation. This structural observation suggests that NINJ1 can form membrane disks, consistent with membrane fragmentation by recombinant NINJ1. Live-cell and super-resolution imaging uncover ring-like structures on the plasma membrane that are released into the culture supernatant. Released NINJ1 encircles a membrane inside, as shown by lipid staining. Therefore, NINJ1-mediated membrane disk formation is different from gasdermin-mediated pore formation, resulting in membrane loss and plasma membrane rupture.
Copyright © 2024 Elsevier Inc. All rights reserved.
In IScience on 19 April 2024 by Cardanho-Ramos, C., Simões, R. A., et al.
In neurons, it is commonly assumed that mitochondrial replication only occurs in the cell body, after which the mitochondria must travel to the neuron's periphery. However, while mitochondrial DNA replication has been observed to occur away from the cell body, the specific mechanisms involved remain elusive. Using EdU-labelling in mouse primary neurons, we developed a tool to determine the mitochondrial replication rate. Taking of advantage of microfluidic devices, we confirmed that mitochondrial replication also occurs locally in the periphery of neurons. To achieve this, mitochondria require de novo nuclear-encoded, but not mitochondrial-encoded protein translation. Following a proteomic screen comparing synaptic with non-synaptic mitochondria, we identified two elongation factors - eEF1A1 and TUFM - that were upregulated in synaptic mitochondria. We found that mitochondrial replication is impaired upon the downregulation of eEF1A1, and this is particularly relevant in the periphery of neurons.© 2024 The Authors.
-
Cell Biology
-
Neuroscience
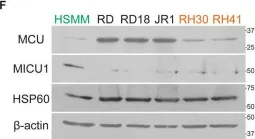
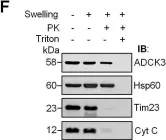
In Cell Death Dis on 30 April 2022 by Chiu, H. Y., Loh, A. H. P., et al.
Fig.1.F

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 12
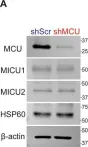
In Cell Death Dis on 30 April 2022 by Chiu, H. Y., Loh, A. H. P., et al.
Fig.2.A

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 12
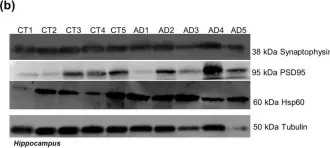
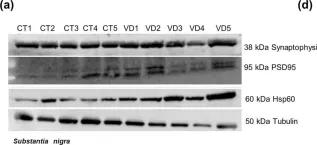
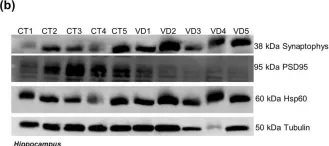
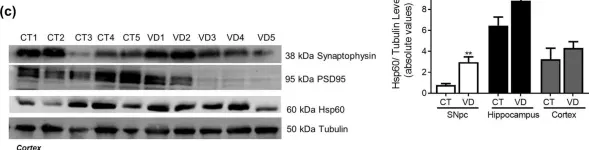
In Sci Rep on 4 August 2020 by Esteves, A. R. & Cardoso, S. M.
Fig.5.C

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 12
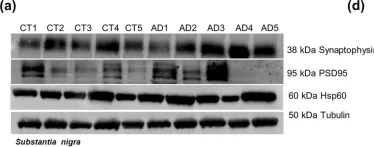
In Sci Rep on 4 August 2020 by Esteves, A. R. & Cardoso, S. M.
Fig.5.A

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 12
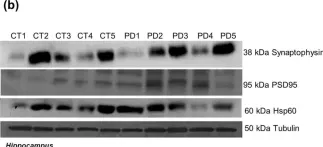
In Sci Rep on 4 August 2020 by Esteves, A. R. & Cardoso, S. M.
Fig.5.B

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 12
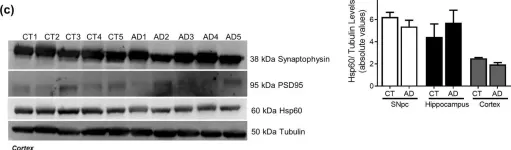
In Sci Rep on 4 August 2020 by Esteves, A. R. & Cardoso, S. M.
Fig.10.A

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 12
In Sci Rep on 4 August 2020 by Esteves, A. R. & Cardoso, S. M.
Fig.10.B

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 12
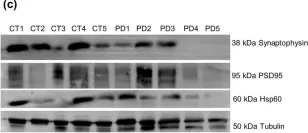
In Sci Rep on 4 August 2020 by Esteves, A. R. & Cardoso, S. M.
Fig.10.C

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 12
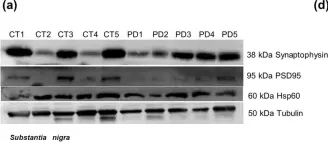
In Sci Rep on 4 August 2020 by Esteves, A. R. & Cardoso, S. M.
Fig.15.A

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 12
In Sci Rep on 4 August 2020 by Esteves, A. R. & Cardoso, S. M.
Fig.15.B

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 12
In Sci Rep on 4 August 2020 by Esteves, A. R. & Cardoso, S. M.
Fig.15.C

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 12
In PLoS One on 13 February 2016 by Cullen, J. K., Abdul Murad, N., et al.
Fig.1.F

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 12