Demodex mites are commensal parasites of hair follicles (HFs). Normally asymptomatic, inflammatory outgrowth of mites can accompany malnutrition, immune dysfunction, and aging, but mechanisms restricting Demodex outgrowth are not defined. Here, we show that control of mite HF colonization in mice required group 2 innate lymphoid cells (ILC2s), interleukin-13 (IL-13), and its receptor, IL-4Ra-IL-13Ra1. HF-associated ILC2s elaborated IL-13 that attenuated HFs and epithelial proliferation at anagen onset; in their absence, Demodex colonization led to increased epithelial proliferation and replacement of gene programs for repair by aberrant inflammation, leading to the loss of barrier function and HF exhaustion. Humans with rhinophymatous acne rosacea, an inflammatory condition associated with Demodex, had increased HF inflammation with decreased type 2 cytokines, consistent with the inverse relationship seen in mice. Our studies uncover a key role for skin ILC2s and IL-13, which comprise an immune checkpoint that sustains cutaneous integrity and restricts pathologic infestation by colonizing HF mites.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Product Citations: 9
Innate type 2 immunity controls hair follicle commensalism by Demodex mites.
In Immunity on 11 October 2022 by Ricardo-Gonzalez, R. R., Kotas, M. E., et al.
-
IHC
-
Mus musculus (House mouse)
-
Immunology and Microbiology
Preprint on BioRxiv : the Preprint Server for Biology on 15 February 2022 by Wu, Y., Konaté, M. M., et al.
h4>ABSTRACT/h4> Previously, we demonstrated that pro-inflammatory cytokines enhance dual oxidase 2 (DUOX2)-dependent production of reactive oxygen species by human pancreatic ductal carcinoma (PDAC) cells, and that DUOX2 expression is significantly increased in patients with early stages of PDAC. In genetically-engineered mouse models of PDAC, dexamethasone (Dex) decreases formation of pancreatic intraepithelial neoplasia (PanIn) foci as well as PDAC invasiveness. Herein, we report that Dex, in a concentration- and time-dependent fashion, inhibited pro-inflammatory cytokine (IFN-γ/LPS/IL-17A/IL-4)-mediated enhancement of DUOX2 expression in BxPC-3, CFPAC-1, and AsPC-1 human PDAC cell lines, as well as DUOX2–induced DNA damage. The inhibitory effects of Dex were abolished by pre-treatment with the Dex antagonist RU-486. Examination of the human DUOX2 promoter in silico revealed a putative negative glucocorticoid receptor (GR) binding element (IRnGRE). Western analysis, using nuclear extracts from Dex-treated PDAC cells, demonstrated that Dex activated the glucocorticoid receptor in PDAC cell nuclei in the presence of certain co-repressors, such as NCoR-1/2 and histone deacetylases (HDAC1, 2, and 3). Dex produced no anti-proliferative effects on PDAC cells in vitro . However, Dex significantly decreased the growth of BxPC-3 xenografts while decreasing inflammatory and immune cell infiltration of the microenvironment, as well as the mRNA expression of DUOX2 and VEGF-A, in BxPC-3 tumors. In contrast, Dex had no effect on the growth of xenografts developed from MIA-PaCa cells that are unresponsive to pro-inflammatory cytokines in culture. In summary, these studies suggest that suppression of inflammation-related DUOX2 expression by Dex could diminish the oxidative milieu supporting PDAC growth and development.
-
IHC
-
Cancer Research
-
Genetics
In The Journal of Immunology on 1 November 2019 by Wu, Y., Konaté, M. M., et al.
Dual oxidase 2 (DUOX2) generates H2O2 that plays a critical role in both host defense and chronic inflammation. Previously, we demonstrated that the proinflammatory mediators IFN-γ and LPS enhance expression of DUOX2 and its maturation factor DUOXA2 through STAT1- and NF-κB‒mediated signaling in human pancreatic cancer cells. Using a panel of colon and pancreatic cancer cell lines, we now report the induction of DUOX2/DUOXA2 mRNA and protein expression by the TH2 cytokine IL-4. IL-4 activated STAT6 signaling that, when silenced, significantly decreased induction of DUOX2. Furthermore, the TH17 cytokine IL-17A combined synergistically with IL-4 to increase DUOX2 expression in both colon and pancreatic cancer cells mediated, at least in part, by signaling through NF-κB. The upregulation of DUOX2 was associated with a significant increase in the production of extracellular H2O2 and DNA damage-as indicated by the accumulation of 8-oxo-dG and γH2AX-which was suppressed by the NADPH oxidase inhibitor diphenylene iodonium and a DUOX2-specific small interfering RNA. The clinical relevance of these experiments is suggested by immunohistochemical, microarray, and quantitative RT-PCR studies of human colon and pancreatic tumors demonstrating significantly higher DUOX2, IL-4R, and IL-17RA expression in tumors than in adjacent normal tissues; in pancreatic adenocarcinoma, increased DUOX2 expression is adversely associated with overall patient survival. These data suggest a functional association between DUOX2-mediated H2O2 production and induced DNA damage in gastrointestinal malignancies.
-
WB
-
Homo sapiens (Human)
-
Cancer Research
-
Genetics
-
Immunology and Microbiology
In Journal of Autoimmunity on 1 July 2019 by Didonna, A., Cantó, E., et al.
The molecular events underlying the transition from initial inflammatory flares to the progressive phase of multiple sclerosis (MS) remain poorly understood. Here, we report that the microtubule-associated protein (MAP) Tau exerts a gender-specific protective function on disease progression in the MS model experimental autoimmune encephalomyelitis (EAE). A detailed investigation of the autoimmune response in Tau-deficient mice excluded a strong immunoregulatory role for Tau, suggesting that its beneficial effects are presumably exerted within the central nervous system (CNS). Spinal cord transcriptomic data show increased synaptic dysfunctions and alterations in the NF-kB activation pathway upon EAE in Tau-deficient mice as compared to wildtype animals. We also performed the first comprehensive characterization of Tau post-translational modifications (PTMs) in the nervous system upon EAE. We report that the methylation levels of the conserved lysine residue K306 are significantly decreased in the chronic phase of the disease. By combining biochemical assays and molecular dynamics (MD) simulations, we demonstrate that methylation at K306 decreases the affinity of Tau for the microtubule network. Thus, the down-regulation of this PTM might represent a homeostatic response to enhance axonal stability against an autoimmune CNS insult. The results, altogether, position Tau as key mediator between the inflammatory processes and neurodegeneration that seems to unify many CNS diseases.
Copyright © 2019 Elsevier Ltd. All rights reserved.
-
Biochemistry and Molecular biology
In Oncotarget on 13 June 2017 by Liu, H., Antony, S., et al.
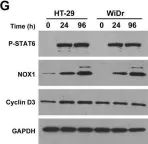
Human colon cancers express higher levels of NADPH oxidase 1 [NOX1] than adjacent normal epithelium. It has been suggested that reactive oxygen species [ROS] derived from NOX1 contribute to DNA damage and neoplastic transformation in the colon, particularly during chronic inflammatory stress. However, the mechanism(s) underlying increased NOX1 expression in malignant tumors or chronic inflammatory states involving the intestine are poorly characterized. We examined the effects of two pro-inflammatory cytokines, IL-4 and IL-13, on the regulation of NOX1. NOX1 expression was increased 4- to 5-fold in a time- and concentration-dependent manner by both cytokines in human colon cancer cell lines when a functional Type II IL-4 receptor was present. Increased NOX1 transcription following IL-4/IL-13 exposure was mediated by JAK1/STAT6 signaling, was associated with a ROS-related inhibition of protein tyrosine phosphatase activity, and was dependent upon activation and specific binding of GATA3 to the NOX1 promoter. NOX1-mediated ROS production increased cell cycle progression through S-phase leading to a significant increase in cellular proliferation. Evaluation of twenty pairs of surgically-resected colon cancers and their associated uninvolved adjacent colonic epithelium demonstrated a significant increase in the active form of NOX1, NOX1-L, in tumors compared to normal tissues, and a significant correlation between the expression levels of NOX1 and the Type II IL-4 receptor in tumor and the uninvolved colon. These studies imply that NOX1 expression, mediated by IL-4/IL-13, could contribute to an oxidant milieu capable of supporting the initiation or progression of colonic cancer, suggesting a role for NOX1 as a therapeutic target.
-
WB
-
Cancer Research
In Oncotarget on 13 June 2017 by Liu, H., Antony, S., et al.
Fig.3.G

-
WB
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 1