The microtubule-associated protein Tau (encoded by the MAPT gene) is linked to a family of neurodegenerative disorders defined as tauopathies, which are characterized by its brain accumulation in neurofibrillary tangles and neuropil threads. Newly described Tau functions comprise DNA protection, chromatin remodeling, p53 regulation and cell fate modulation, suggesting a role of Tau in oncogenesis. Bioinformatic-supported characterization of Tau in cancer reveals robust expression in bone cancer cells, in particular Ewing sarcoma (EwS) cell lines. EwS is an aggressive cancer caused by a fusion of members of the FET and ETS gene families, primarily EWSR1::FLI1. Here we found that MAPT is a EWSR1::ETS target gene and that higher Tau expression in EwS cells inhibited their migratory and invasive behavior, consistent with a more immobile and proliferative phenotype observed in EwS. Indeed, we report that high Tau expression is associated with improved overall survival of EwS patients. We also show that the sessile but proliferative phenotype of EWSR1::ETS-high cells may result from a modulatory role of Tau on focal adhesion to extracellular matrix proteins. Our data highlight the utility of determining Tau expression as a prognostic factor in EwS as well as the opportunity to target Tau expression as an innovative EwS therapy.
© 2025. The Author(s).
Product Citations: 15
High Tau expression correlates with reduced invasion and prolonged survival in Ewing sarcoma.
In Cell Death Discovery on 3 May 2025 by Cidre-Aranaz, F., Magrin, C., et al.
-
WB
-
Homo sapiens (Human)
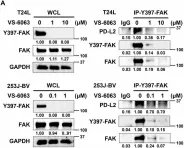
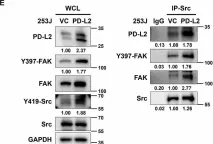
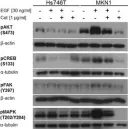
SPHK1 promotes bladder cancer metastasis via PD-L2/c-Src/FAK signaling cascade.
In Cell Death & Disease on 16 September 2024 by Kao, W. H., Liao, L. Z., et al.
SPHK1 (sphingosine kinase type 1) is characterized as a rate-limiting enzyme in sphingolipid metabolism to phosphorylate sphingosine into sphingosine-1-phosphate (S1P) that can bind to S1P receptors (S1PRs) to initiate several signal transductions leading to cell proliferation and survival of normal cell. Many studies have indicated that SPHK1 is involved in several types of cancer development, however, a little is known in bladder cancer. The TCGA database analysis was utilized for analyzing the clinical relevance of SPHK1 in bladder cancer. Through CRISPR/Cas9 knockout (KO) and constitutive activation (CA) strategies on SPHK1 in the bladder cancer cells, we demonstrated the potential downstream target could be programmed cell death 1 ligand 2 (PD-L2). On the other hand, we demonstrated that FDA-approved SPHK1 inhibitor Gilenya® (FTY720) can successfully suppress bladder cancer metastasis by in vitro and in vivo approaches. This finding indicated that SPHK1 as a potent therapeutic target for metastatic bladder cancer by dissecting the mechanism of action, SPHK1/S1P-elicited Akt/β-catenin activation promoted the induction of PD-L2 that is a downstream effector in facilitating bladder cancer invasion and migration. Notably, PD-L2 interacted with c-Src that further activates FAK. Here, we unveil the clinical relevance of SPHK1 in bladder cancer progression and the driver role in bladder cancer metastasis. Moreover, we demonstrated the inhibitory effect of FDA-approved SPHK1 inhibitor FTY720 on bladder cancer metastasis from both in vitro and in vivo models.
© 2024. The Author(s).
-
WB
-
Cancer Research
-
Cell Biology
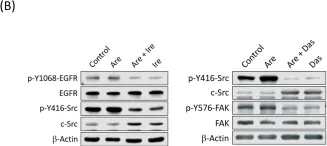
In Virulence on 1 December 2023 by Farag, S. I., Francis, M. K., et al.
Translocon pores formed in the eukaryotic cell membrane by a type III secretion system facilitate the translocation of immune-modulatory effector proteins into the host cell interior. The YopB and YopD proteins produced and secreted by pathogenic Yersinia spp. harboring a virulence plasmid-encoded type III secretion system perform this pore-forming translocator function. We had previously characterized in vitro T3SS function and in vivo pathogenicity of a number of strains encoding sited-directed point mutations in yopD. This resulted in the classification of mutants into three different classes based upon the severity of the phenotypic defects. To investigate the molecular and functional basis for these defects, we explored the effectiveness of RAW 264.7 cell line to respond to infection by representative YopD mutants of all three classes. Signature cytokine profiles could separate the different YopD mutants into distinct categories. The activation and suppression of certain cytokines that function as central innate immune response modulators correlated well with the ability of mutant bacteria to alter anti-phagocytosis and programmed cell death pathways. These analyses demonstrated that sub-optimal translocon pores impact the extent and magnitude of host cell responsiveness, and this limits the capacity of pathogenic Yersinia spp. to fortify against attack by both early and late arms of the host innate immune response.
-
Immunology and Microbiology
In Cells on 17 October 2021 by Pai, F. C., Huang, H. W., et al.
Malignant glioma is one of the most lethal cancers with rapid progression, high recurrence, and poor prognosis in the central nervous system. Fatty acid-binding protein 6 (FABP6) is a bile acid carrier protein that is overexpressed in colorectal cancer. This study aimed to assess the involvement of FABP6 expression in the progression of malignant glioma. Immunohistochemical analysis revealed that FABP6 expression was higher in glioma than in normal brain tissue. After the knockdown of FABP6, a decrease in the migration and invasion abilities of glioma cells was observed. The phosphorylation of the myosin light chain was inhibited, which may be associated with migration ability. Moreover, expression levels of invasion-related proteins, matrix metalloproteinase-2 (MMP-2) and cathepsin B, were reduced. Furthermore, tube formation was inhibited in the human umbilical vein endothelial cells with a decreased concentration of vascular endothelial growth factor (VEGF) in the conditioned medium after the knockdown of FABP6. The phosphorylation of the extracellular signal-regulated kinase (ERK), c-Jun NH2-terminal kinase (JNK), and p65 were also decreased after FABP6 reduction. Finally, the bioluminescent images and immunostaining of MMP-2, cluster of differentiation 31 (CD31), and the VEGF receptor 1 (VEGFR1) revealed attenuated tumor progression in the combination of the FABP6-knocked-down and temozolomide (TMZ)-treated group in an orthotopic xenograft mouse tumor model. This is the first study that revealed the impact of FABP6 on the invasion, angiogenesis, and progression of glioma. The results of this study show that FABP6 may be a potential therapeutic target combined with TMZ for malignant gliomas.
-
Cancer Research
-
Cell Biology
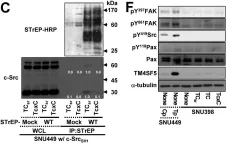
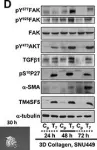
In Theranostics on 3 August 2021 by Song, H. E., Lee, Y., et al.
Active c-Src non-receptor tyrosine kinase localizes to the plasma membrane via N-terminal lipid modification. Membranous c-Src causes cancer initiation and progression. Even though transmembrane 4 L six family member 5 (TM4SF5), a tetraspan(in), can be involved in this mechanism, the molecular and structural influence of TM4SF5 on c-Src remains unknown. Methods: Here, we investigated molecular and structural details by which TM4SF5 regulated c-Src devoid of its N-terminus and how cell-penetrating peptides were able to interrupt c-Src activation via interference of c-Src-TM4SF5 interaction in hepatocellular carcinoma models. Results: The TM4SF5 C-terminus efficiently bound the c-Src SH1 kinase domain, efficiently to the inactively-closed form. The complex involved protein tyrosine phosphatase 1B able to dephosphorylate Tyr530. The c-Src SH1 domain alone, even in a closed form, bound TM4SF5 to cause c-Src Tyr419 and FAK Y861 phosphorylation. Homology modeling and molecular dynamics simulation studies predicted the directly interfacing residues, which were further validated by mutational studies. Cell penetration of TM4SF5 C-terminal peptides blocked the interaction of TM4SF5 with c-Src and prevented c-Src-dependent tumor initiation and progression in vivo. Conclusions: Collectively, these data demonstrate that binding of the TM4SF5 C-terminus to the kinase domain of inactive c-Src leads to its activation. Because this binding can be abolished by cell-penetrating peptides containing the TM4SF5 C-terminus, targeting this direct interaction may be an effective strategy for developing therapeutics that block the development and progression of hepatocellular carcinoma.
© The author(s).
-
WB
-
Cancer Research
In Cell Death Dis on 16 September 2024 by Kao, W. H., Liao, L. Z., et al.
Fig.6.A

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 11
In Cell Death Dis on 16 September 2024 by Kao, W. H., Liao, L. Z., et al.
Fig.6.E

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 11
In Theranostics on 3 August 2021 by Song, H. E., Lee, Y., et al.
Fig.6.E

-
WB
-
Collected and cropped from Theranostics by CiteAb, provided under a CC-BY license
Image 1 of 11
In Theranostics on 3 August 2021 by Song, H. E., Lee, Y., et al.
Fig.6.C

-
WB
-
Collected and cropped from Theranostics by CiteAb, provided under a CC-BY license
Image 1 of 11
In Theranostics on 3 August 2021 by Song, H. E., Lee, Y., et al.
Fig.6.D

-
WB
-
Collected and cropped from Theranostics by CiteAb, provided under a CC-BY license
Image 1 of 11
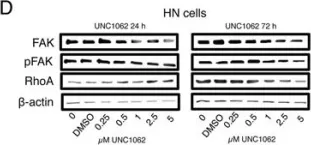
In Toxins (Basel) on 28 March 2019 by Chang, C. H., Chen, M. C., et al.
Fig.4.B

-
WB
-
Collected and cropped from Toxins (Basel) by CiteAb, provided under a CC-BY license
Image 1 of 11
In BMC Cancer on 13 December 2017 by Keller, S., Kneissl, J., et al.
Fig.7.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from BMC Cancer by CiteAb, provided under a CC-BY license
Image 1 of 11
In Oncotarget on 13 October 2017 by Song, D. G., Lee, G. H., et al.
Fig.5.D

-
WB
-
Homo sapiens (Human)
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 11
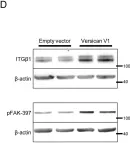
In Oncotarget on 31 May 2016 by von Mässenhausen, A., Sanders, C., et al.
Fig.2.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 11
In Oncotarget on 31 May 2016 by von Mässenhausen, A., Sanders, C., et al.
Fig.6.D

-
WB
-
Homo sapiens (Human)
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 11
In PLoS One on 16 July 2015 by Carthy, J. M., Meredith, A. J., et al.
Fig.3.D

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 11