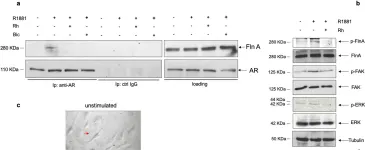
Aging induces a slow and progressive decrease in muscle mass and function, causing sarcopenia. Androgens control muscle trophism and exert important anabolic functions through the binding to the androgen receptor. Therefore, analysis of the androgen receptor-mediated actions in skeletal muscle might provide new hints for a better understanding of sarcopenia pathogenesis. In this study, we report that expression of the androgen receptor in skeletal muscle biopsies from 20 subjects is higher in young, as compared with old subjects. Co-immunoprecipitation experiments reveal that the androgen receptor is complexed with filamin A mainly in young, that in old subjects. Therefore, we have in depth analyzed the role of such complex using C2C12 myoblasts that express a significant amount of the androgen receptor. In these cells, hormone stimulation rapidly triggers the assembly of the androgen receptor/filamin A complex. Such complex prevents the senescence induced by oxidative stress in C2C12 cells, as disruption of the androgen receptor/filamin A complex by Rh-2025u stapled peptide re-establishes the senescent phenotype in C2C12 cells. Simultaneously, androgen stimulation of C2C12 cells rapidly triggers the activation of various signaling effectors, including Rac1, focal adhesion kinase, and mitogen-activated kinases. Androgen receptor blockade by bicalutamide or perturbation of androgen receptor/filamin A complex by Rh-2025u stapled peptide both reverse the hormone activation of signaling effectors. These findings further reinforce the role of the androgen receptor and its extranuclear partners in the rapid hormone signaling that controls the functions of C2C12 cells. Further investigations are needed to promote clinical interventions that might ameliorate muscle cell function as well the clinical outcome of age-related frailty.
© 2023. The Author(s).
Product Citations: 23
Filamin A cooperates with the androgen receptor in preventing skeletal muscle senescence.
In Cell Death Discovery on 2 December 2023 by Di Donato, M., Moretti, A., et al.
-
WB
-
Endocrinology and Physiology
In Oncology Reports on 1 March 2023 by Kim, J. E., Lee, S. K., et al.
Colon cancer is one of the most frequent malignant neoplasms worldwide. Epidemiological studies suggested that the development of colon cancer can be prevented by plant‑derived ingredients. In the present study, the chemopreventive activity of buddlejasaponin IV (BS‑IV), isolated from the aerial part of Pleurospermum kamtschaticum, was investigated using cell viability, DNA fragmentation, caspase‑3 activity, anoikis, cell adhesion, and flow cytometry assays and a murine lung metastasis model. Protein expression levels were detected by western blotting. Treatment with BS‑IV significantly reduced cell viability and caused DNA fragmentation in HT‑29 human colorectal cancer cells. BS‑IV increased the ratio of Bax to Bcl‑2 by significantly inhibiting Bcl‑2 expression levels. BS‑IV reduced expression levels of procaspase‑9, procaspase‑3, and full‑length poly (ADP‑ribose) polymerase (PARP) and increased cleaved PARP and nonsteroidal anti‑inflammatory drug activated gene‑1 expression levels and caspase‑3 activity. In addition, BS‑IV decreased the attachment of HT‑29 cells to the extracellular matrix proteins collagen type I and IV and downregulated cell surface expression of α2β1 integrin by inhibiting its glycosylation. BS‑IV also reduced the expression and phosphorylation levels of focal adhesion kinase (FAK) and Akt, and the reduced FAK and Akt levels were rescued by treatment with a caspase‑3 inhibitor Z‑VAD‑FMK. Furthermore, orally administered BS‑IV inhibited the formation of tumor nodules in Balb/C mice intravenously injected with CT‑26 murine colorectal cancer cells. Collectively, these findings indicated that BS‑IV induces apoptosis via the mitochondrial‑dependent pathway by increasing the ratio of Bax to Bcl‑2 and activating caspases. BS‑IV also induces anoikis by inhibiting α2β1 integrin‑mediated cell adhesion and signaling and inhibits the lung metastasis of colon cancer cells. Therefore, BS‑IV may serve as a promising cancer chemopreventive agent.
-
Cancer Research
-
Cell Biology
In Cancer Science on 1 June 2022 by Liu, M., Yan, R., et al.
Metastasis is the main cause of cancer patients' death despite tremendous efforts invested in developing the related molecular mechanisms. During cancer cell migration, cells undergo dynamic regulation of filopodia, focal adhesion, and endosome trafficking. Cdc42 is imperative for maintaining cell morphology and filopodia, regulating cell movement. Integrin beta1 activates on the endosome, the majority of which distributes itself on the plasma membrane, indicating that endocytic trafficking is essential for this activity. In cancers, high expression of lysosome-associated protein transmembrane 4B (LAPTM4B) is associated with poor prognosis. LAPTM4B-35 has been reported as displaying plasma membrane distribution and being associated with cancer cell migration. However, the detailed mechanism of its isoform-specific distribution and whether it relates to cell migration remain unknown. Here, we first report and quantify the filopodia localization of LAPTM4B-35: mechanically, that specific interaction with Cdc42 promoted its localization to the filopodia. Furthermore, our data show that LAPTM4B-35 stabilized filopodia and regulated integrin beta1 recycling via interaction and cotrafficking on the endosome. In our zebrafish xenograft model, LAPTM4B-35 stimulated the formation and dynamics of focal adhesion, further promoting cancer cell dissemination, whereas in skin cancer patients, LAPTM4B level correlated with poor prognosis. In short, this study establishes an insight into the mechanism of LAPTM4B-35 filopodia distribution, as well as into its biological effects and its clinical significance, providing a novel target for cancer therapeutics development.
© 2022 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
-
Cancer Research
In International Journal of Molecular Sciences on 1 January 2022 by Yeh, L. T., Lin, C. W., et al.
Osteosarcoma is a highly common malignant bone tumor. Its highly metastatic properties are the leading cause of mortality for cancer. Niclosamide, a salicylanilide derivative, is an oral antihelminthic drug of known anticancer potential. However, the effect of niclosamide on osteosarcoma cell migration, invasion and the mechanisms underlying have not been fully clarified. Therefore, this study investigated niclosamide's underlying pathways and antimetastatic effects on osteosarcoma. In this study, U2OS and HOS osteosarcoma cell lines were treated with niclosamide and then subjected to assays for determining cell migration ability. The results indicated that niclosamide, at concentrations of up to 200 nM, inhibited the migration and invasion of human osteosarcoma U2OS and HOS cells and repressed the transforming growth factor beta-induced protein (TGFBI) expression of U2OS cells, without cytotoxicity. After TGFBI knockdown occurred, cellular migration and invasion behaviors of U2OS cells were significantly reduced. Moreover, niclosamide significantly decreased the phosphorylation of ERK1/2 in U2OS cells and the combination treatment of the MEK inhibitor (U0126) and niclosamide resulted in the intensive inhibition of the TGFBI expression and the migratory ability in U2OS cells. Therefore, TGFBI derived from osteosarcoma cells via the ERK pathway contributed to cellular migration and invasion and niclosamide inhibited these processes. These findings indicate that niclosamide may be a powerful preventive agent against the development and metastasis of osteosarcoma.
-
WB
-
Homo sapiens (Human)
-
Cancer Research
In Journal of Cellular and Molecular Medicine on 1 October 2021 by Chen, P. N., Lin, C. W., et al.
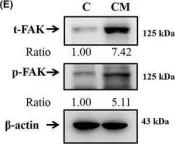
Oral submucous fibrosis (OSF) involves a high risk of malignant transformation and has been implicated in oral cancer. Limited studies have been conducted on the role of OSF in relation to the invasive capabilities and epithelial-mesenchymal transition (EMT) in oral cancer. Herein, we investigated the effects of OSF on the microenvironment of human oral cancer cells. The results showed that the conditioned medium (CM) of fibrotic buccal mucosal fibroblasts (fBMFs) strongly induced the invasion of oral cancer cells and increased the activities of matrix metalloproteinase-2. OSF significantly induced the EMT in oral cancer cells and downregulated epithelial markers, such as E-cadherin, but significantly elevated vimentin, fibronectin, N-cadherin, RhoA, Rac-1 and FAK. Insulin-like growth factor-1 (IGF-1) was elevated in OSF. The protein levels of the IGF-1R were upregulated specifically in fBMF CM treatment for oral cancer cells, and the IGFR gene was confirmed by The Cancer Genome Atlas patient transcriptome data. The Kaplan-Meier curve analysis revealed that patients with oral squamous cell carcinoma and high IGFR expression levels had poorer 5-year survival than those with low IGFR expression (p = 0.004). The fBMF-stimulated EMT cell model may recapture some of the molecular changes during EMT progression in clinical patients with oral cancer.
© 2021 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
-
WB
-
Biochemistry and Molecular biology
-
Cancer Research
In Cell Death Discov on 2 December 2023 by Di Donato, M., Moretti, A., et al.
Fig.5.A

-
WB
-
Collected and cropped from Cell Death Discov by CiteAb, provided under a CC-BY license
Image 1 of 3
In J Cell Mol Med on 1 October 2021 by Chen, P. N., Lin, C. W., et al.
Fig.4.E

-
WB
-
Collected and cropped from J Cell Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 3
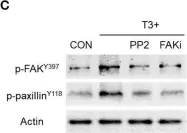
In Front Endocrinol (Lausanne) on 23 March 2019 by Uzair, I. D., Conte Grand, J., et al.
Fig.3.C

-
WB
-
Collected and cropped from Front Endocrinol (Lausanne) by CiteAb, provided under a CC-BY license
Image 1 of 3