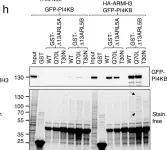
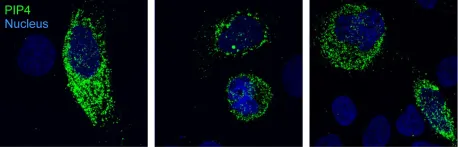
ARL5 is a member of the ARF family of small GTPases that is recruited to the trans-Golgi network (TGN) by another ARF-family member, ARFRP1, in complex with the transmembrane protein SYS1. ARL5 recruits its effector, the multisubunit tethering complex GARP, to promote SNARE-dependent fusion of endosome-derived retrograde transport carriers with the TGN. To further investigate the function of ARL5, we sought to identify additional effectors. Using proximity biotinylation and protein interaction assays, we found that the armadillo-repeat protein ARMH3 (C10orf76) binds to active, but not inactive, ARL5, and that it is recruited to the TGN in a SYS1-ARFRP1-ARL5-dependent manner. Unlike GARP, ARMH3 is not required for the retrograde transport of various cargo proteins. Instead, ARMH3 functions to activate phosphatidylinositol 4-kinase IIIβ (PI4KB), accounting for the main pool of phosphatidylinositol 4-phosphate (PI4P) at the TGN. This function contributes to recruitment of the oncoprotein GOLPH3 and glycan modifications at the TGN. These studies thus identify the SYS1-ARFRP1-ARL5-ARMH3 axis as a regulator of PI4KB-dependent generation of PI4P at the TGN.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Product Citations: 5
ARMH3 is an ARL5 effector that promotes PI4KB-catalyzed PI4P synthesis at the trans-Golgi network.
In Nature Communications on 23 November 2024 by Ishida, M., Golding, A. E., et al.
-
WB
-
ICC-IF
-
IF
-
Homo sapiens (Human)
-
Cell Biology
Phosphatidylinositol 4-kinase III beta regulates cell shape, migration, and focal adhesion number.
In Molecular Biology of the Cell on 1 August 2020 by Bilodeau, P., Jacobsen, D., et al.
Cell shape is regulated by cell adhesion and cytoskeletal and membrane dynamics. Cell shape, adhesion, and motility have a complex relationship and understanding them is important in understanding developmental patterning and embryogenesis. Here we show that the lipid kinase phosphatidylinositol 4-kinase III beta (PI4KIIIβ) regulates cell shape, migration, and focal adhesion (FA) number. PI4KIIIβ generates phosphatidylinositol 4-phosphate (PI4P) from phosphatidylinositol and is highly expressed in a subset of human breast cancers. PI4KIIIβ and the PI4P it generates regulate a variety of cellular functions, ranging from control of Golgi structure, fly fertility, and Akt signaling. Here, we show that loss of PI4KIIIβ expression decreases cell migration and alters cell shape in NIH3T3 fibroblasts. The changes are accompanied by an increase in the number of FA in cells lacking PI4KIIIβ. Furthermore, we find that PI4P-containing vesicles move to the migratory leading edge during migration and that some of these vesicles tether to and fuse with FA. Fusion is associated with FA disassembly. This suggests a novel regulatory role for PI4KIIIβ and PI4P in cell adhesion and cell shape maintenance.
-
WB
-
Mus musculus (House mouse)
-
Cell Biology
Preprint on BioRxiv : the Preprint Server for Biology on 11 February 2020 by MacDonald, S. A., Harding, K., et al.
h4>ABSTRACT/h4> Endosomes are now recognized as important sites for regulating signal transduction. Here we show that the lipid kinase phosphatidylinositol 4-kinase III beta (PI4KIIIβ) regulates both endocytic kinetics and receptor signaling in breast cancer cells. PI4KIIIβ generates phosphatidylinositol 4-phosphate from phosphatidylinositol and is highly expressed in a subset of breast cancers. However, the molecular mechanism by which PI4KIIIβ promotes breast cancer is unclear. We demonstrate that ectopic PI4KIIIβ expression increases the rates of both endocytic internalization and recycling. PI4KIIIβ deletion reduces endocytic kinetics accompanied by a concomitant decrease in activity of the Rab11a GTPase, a protein required for endocytic function. Finally, we find that PI4KIIIβ activates IGF-IRβ signaling dependent on endosome function. Regulation of endocytic function by PI4KIIIβ is independent of its kinase activity but requires interaction with the Rab11a. This suggests that PI4KIIIβ controls endosomal kinetics and signaling by directly modulating Rab11a function. Our work suggests a novel regulatory role for PI4KIIIβ in endosome function and plasma membrane receptor signaling.
-
Cancer Research
A high-throughput pipeline for validation of antibodies.
In Nature Methods on 1 November 2018 by Sikorski, K., Mehta, A., et al.
Western blotting (WB) is widely used to test antibody specificity, but the assay has low throughput and precision. Here we used preparative gel electrophoresis to develop a capture format for WB. Fractions with soluble, size-separated proteins facilitated parallel readout with antibody arrays, shotgun mass spectrometry (MS) and immunoprecipitation followed by MS (IP-MS). This pipeline provided the means for large-scale implementation of antibody validation concepts proposed by an international working group on antibody validation (IWGAV).
-
PAGE-MAP
-
Homo sapiens (Human)
In PLoS Pathogens on 14 March 2012 by Bianco, A., Reghellin, V., et al.
4-anilino quinazolines have been identified as inhibitors of HCV replication. The target of this class of compounds was proposed to be the viral protein NS5A, although unequivocal proof has never been presented. A 4-anilino quinazoline moiety is often found in kinase inhibitors, leading us to formulate the hypothesis that the anti-HCV activity displayed by these compounds might be due to inhibition of a cellular kinase. Type III phosphatidylinositol 4-kinase α (PI4KIIIα) has recently been identified as a host factor for HCV replication. We therefore evaluated AL-9, a compound prototypical of the 4-anilino quinazoline class, on selected phosphatidylinositol kinases. AL-9 inhibited purified PI4KIIIα and, to a lesser extent, PI4KIIIβ. In Huh7.5 cells, PI4KIIIα is responsible for the phosphatidylinositol-4 phosphate (PI4P) pool present in the plasma membrane. Accordingly, we observed a gradual decrease of PI4P in the plasma membrane upon incubation with AL-9, indicating that this agent inhibits PI4KIIIα also in living cells. Conversely, AL-9 did not affect the level of PI4P in the Golgi membrane, suggesting that the PI4KIIIβ isoform was not significantly inhibited under our experimental conditions. Incubation of cells expressing HCV proteins with AL-9 induced abnormally large clusters of NS5A, a phenomenon previously observed upon silencing PI4KIIIα by RNA interference. In light of our findings, we propose that the antiviral effect of 4-anilino quinazoline compounds is mediated by the inhibition of PI4KIIIα and the consequent depletion of PI4P required for the HCV membranous web. In addition, we noted that HCV has a profound effect on cellular PI4P distribution, causing significant enrichment of PI4P in the HCV-membranous web and a concomitant depletion of PI4P in the plasma membrane. This observation implies that HCV--by recruiting PI4KIIIα in the RNA replication complex--hijacks PI4P metabolism, ultimately resulting in a markedly altered subcellular distribution of the PI4KIIIα product.
-
IF
-
ICC-IF
-
Homo sapiens (Human)
-
Biochemistry and Molecular biology
-
Cell Biology
-
Immunology and Microbiology
In Nat Commun on 23 November 2024 by Ishida, M., Golding, A. E., et al.
Fig.4.H

-
WB
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 4
In Nat Commun on 23 November 2024 by Ishida, M., Golding, A. E., et al.
Fig.6.W

-
ICC-IF
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 4
In PLoS Pathog on 14 March 2012 by Bianco, A., Reghellin, V., et al.
Fig.7.A

-
ICC-IF
-
Homo sapiens (Human)
Collected and cropped from PLoS Pathog by CiteAb, provided under a CC-BY license
Image 1 of 4
In PLoS Pathog on 14 March 2012 by Bianco, A., Reghellin, V., et al.
Fig.9.A

-
ICC-IF
-
Homo sapiens (Human)
Collected and cropped from PLoS Pathog by CiteAb, provided under a CC-BY license
Image 1 of 4