Traumatic brain injury (TBI) is a complex medical condition affecting people globally. Hydrogen sulfide (H2S) is a recently discovered gaseous mediator and is dysregulated in the brain after TBI. Sodium hydrogen sulfide (NaHS), a known donor of H2S, is beneficial in various biological processes involving aging and diseases, including injury. It is neuroprotective against oxidative stress, neuroinflammation, and other secondary injury processes. However, the NaHS-H2S system has not been investigated as a regulator of injury-mediated synaptic plasticity proteins and the underlying mechanisms after TBI.
We developed a model of TBI in Swiss albino mice to study the effects of exogenous H2S, administered as NaHS. We assessed cognitive function (Barnes maze and novel object recognition) and motor function (rotarod). Brain tissue was analysed with ELISA, qRT-PCR, immunoblotting, Golgi-cox staining, and immunofluorescence.
NaHS administration restored the injury-caused decline in H2S levels. Injury-mediated oxidative stress parameters were improved following NaHS. It down-regulated TBI biomarkers, ameliorated the synaptic marker proteins, and improved cognitive and motor deficits. These changes were accompanied by enhanced dendritic arborization and spine number. Restoration of N-methyl D-aspartate receptor subunits and diminished glutamate and calcium levels, along with marked changes in microtubule-associated protein 2 A and calcium/calmodulin-dependent protein kinase II, formed the basis of the underlying mechanism(s).
Our findings suggest that NaHS could have therapeutic activity against TBI, as it ameliorated cognitive and motor deficits caused by changes in synaptic plasticity proteins and dendritic arborisation, in our model.
© 2024 British Pharmacological Society.
Product Citations: 40
In British Journal of Pharmacology on 1 March 2025 by Nasir, F., Yadav, P., et al.
-
Immunology and Microbiology
-
Neuroscience
-
Pharmacology
Gpnmb Defines a Phagocytic State of Microglia Linked to Neuronal Loss in Prion Disease
Preprint on BioRxiv : the Preprint Server for Biology on 12 February 2025 by Caredio, D., Mariutti, G., et al.
The reaction of different cell types to prion infections is highly heterogeneous. While neurons experience spine retraction and eventually death, astrocytes and microglia undergo strong activation and proliferation. Here we analyzed the cell-type specific responses to prion diseases by establishing a spatiotemporal transcriptomic atlas of mice infected with RML prion strain. Brain areas with severe neuronal loss, such as thalamus and cerebellum, experienced intense microgliosis. Starting from 30 weeks post-inoculation, we observed the accumulation of a novel microglial subpopulation characterized by strong expression of Gpnmb in these brain regions. The molecular profile of Gpnmb+ microglia reflected a state of enhanced phagocytic activity with upregulation of genes associated with lysosomal function and degradation, including vacuolar ATPase V0 domain subunit d2 (Atp6v0d2) and Galectin-3 (Lgals3). In murine BV2 cells, Gpnmb upregulation was induced by soluble find-me signals released during apoptosis, but not by apoptotic bodies or prion accumulation. Gpnmb ablation in BV2 cells impaired their ability to phagocytose apoptotic cells, underscoring its essential role in maintaining microglial phagocytosis. Our findings define Gpnmb⁺ microglia as a distinct, apoptosis-driven phagocytic state, linking neuronal loss to microglial activation in prion disease. The upregulation of GPNMB in sCJD patients, along with its role in apoptotic clearance and lysosomal function, positions it as both a key regulator of microglial responses and a potential biomarker of disease progression.
-
Neuroscience
Stathmin 1 expression in neuroendocrine and proliferating prostate cancer.
In Discov Oncol on 8 January 2025 by Shi, Y., Yeh, Y. A., et al.
Prostate cancer (PCa) is the second leading cause of cancer-related mortality among men in the United States. While PCa initially responds to androgen deprivation therapy, a significant portion progresses to castration-resistant PCa. Approximately 20-25% of these cases acquire aggressive neuroendocrine (NE) features, ultimately leading to neuroendocrine prostate cancer (NEPC). In this study, we investigated the expression of stathmin 1 (STMN1) across PCa subtypes using bioinformatics, western blotting, and immunohistochemical staining analyses in human and murine models. We found that elevated STMN1 expression correlated with high Gleason Scores, increased cell proliferation, and poor clinical outcomes in PCa patients. Notably, STMN1 expression was significantly higher in NEPC compared to prostate adenocarcinoma, suggesting its role in NEPC progression. Findings from TRAMP tumors, a murine NEPC model, further supported these results. In conclusion, STMN1 expression is elevated in advanced PCa, particularly in NEPC, suggesting its involvement in the progression of aggressive forms of PCa. While STMN1 shows potential as a diagnostic and prognostic marker for aggressive PCa, further studies are necessary to establish its clinical utility.
© 2025. The Author(s).
-
Cancer Research
-
Endocrinology and Physiology
In Cell Rep Methods on 18 November 2024 by Sun, P., Yuan, Y., et al.
Progress has been made in generating spinal cord and trunk derivatives from neuromesodermal progenitors (NMPs). However, maintaining the self-renewal of NMPs in vitro remains a challenge. In this study, we developed a cocktail of small molecules and growth factors that induces human embryonic stem cells to produce self-renewing NMPs (srNMPs) under chemically defined conditions. These srNMPs maintain the state of neuromesodermal progenitors in prolonged culture and have the potential to generate mesodermal cells and neurons, even at the single-cell level. Additionally, suspended srNMP aggregates can spontaneously differentiate into all tissue types of early embryonic trunks. Furthermore, transplanted srNMP-derived muscle satellite cells or progenitors of motor neurons were integrated into skeletal muscle or the spinal cord, respectively, and contributed to regeneration in mouse models. In summary, srNMPs hold great promise for applications in developmental biology and as renewable cell sources for cell therapy for trunk and spinal cord injuries.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
-
Stem Cells and Developmental Biology
In International Journal of Molecular Sciences on 28 October 2024 by Choi, I. A., Yun, J. H., et al.
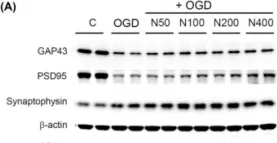
This study explores the neuroprotective effects of neuropeptide FF (NPFF, FLFQPQRFamide) in the context of ischemic injury. Based on transcriptomic analysis in stroke models treated with 5-Aza-dC and task-specific training, we identified significant gene expression changes, particularly involving NPFF. To further explore NPFF's role in promoting neuronal recovery, recombinant NPFF protein (rNPFF) was used in primary mixed cortical cultures subjected to oxygen-glucose deprivation and reoxygenation. Our results demonstrated that rNPFF significantly reduced lactate dehydrogenase release, indicating decreased cellular damage. It also significantly increased the expression of TUJ1 and MAP2, markers of neuronal survival and dendritic integrity. Additionally, rNPFF significantly upregulated key synaptic proteins, including GAP43, PSD95, and synaptophysin, which are essential for synaptic repair and plasticity. Post-injury rNPFF treatment led to a significant upregulation of pro-brain-derived neurotrophic factor (BDNF) and mature BDNF, which play critical roles in neuronal survival, growth, and synaptic plasticity. Moreover, rNPFF activated the protein kinase Cε isoform, Sirtuin 1, and peroxisome proliferator-activated receptor gamma pathways, which are crucial for regulating cellular stress responses, synaptic plasticity, and energy homeostasis, further promoting neuronal survival and recovery. These findings suggest that rNPFF may play a pivotal role in enhancing neuronal survival and synaptic plasticity after ischemic injury, highlighting its potential as a therapeutic target for stroke recovery.
-
WB
In Int J Mol Sci on 28 October 2024 by Choi, I. A., Yun, J. H., et al.
Fig.4.A

-
WB
-
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 2
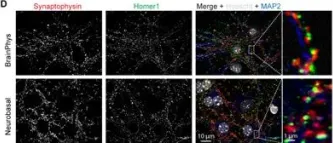
In Front Synaptic Neurosci on 20 May 2020 by Moutin, E., Hemonnot, A. L., et al.
Fig.3.D

-
ICC-IF
-
Mus musculus (House mouse)
Collected and cropped from Front Synaptic Neurosci by CiteAb, provided under a CC-BY license
Image 1 of 2