The assembly and secretion of hepatic very low-density lipoprotein (VLDL) plays pivotal roles in hepatic and plasma lipid homeostasis. Protein disulfide isomerase A1 (PDIA1/P4HB) is a molecular chaperone whose functions are essential for protein folding in the endoplasmic reticulum. Here we investigated the physiological requirement in vivo for PDIA1 in maintaining VLDL assembly and secretion.
Pdia1/P4hb was conditionally deleted in adult mouse hepatocytes and the phenotypes characterized. Mechanistic analyses in primary hepatocytes determined how PDIA1 ablation alters MTTP synthesis and degradation as well as altering synthesis and secretion of Apolipoprotein B (APOB), along with complementary expression of intact PDIA1 vs a catalytically inactivated PDIA1 mutant.
Hepatocyte-specific deletion of Pdia1/P4hb inhibited hepatic MTTP expression and dramatically reduced VLDL production, leading to severe hepatic steatosis and hypolipidemia. Pdia1-deletion did not affect mRNA expression or protein stability of MTTP but rather prevented Mttp mRNA translation. We demonstrate an essential role for PDIA1 in MTTP synthesis and function and show that PDIA1 interacts with APOB in an MTTP-independent manner via its molecular chaperone function to support APOB folding and secretion.
PDIA1 plays indispensable roles in APOB folding, MTTP synthesis and activity to support VLDL assembly. Thus, like APOB and MTTP, PDIA1 is an obligatory component of hepatic VLDL production.
Copyright © 2024. Published by Elsevier GmbH.
Product Citations: 29
In Molecular Metabolism on 1 February 2024 by Chen, Z., Wang, S., et al.
-
Biochemistry and Molecular biology
Bavachin protects against diet-induced hepatic steatosis and obesity in mice.
In Acta Pharmacologica Sinica on 1 July 2023 by Wei, X., Lin, L., et al.
Non-alcoholic fatty liver disease (NAFLD) is a major health concern worldwide, and the incidence of metabolic disorders associated with NAFLD is rapidly increasing because of the obesity epidemic. There are currently no approved drugs that prevent or treat NAFLD. Recent evidence shows that bavachin, a flavonoid isolated from the seeds and fruits of Psoralea corylifolia L., increases the transcriptional activity of PPARγ and insulin sensitivity during preadipocyte differentiation, but the effect of bavachin on glucose and lipid metabolism remains unclear. In the current study we investigated the effects of bavachin on obesity-associated NAFLD in vivo and in vitro. In mouse primary hepatocytes and Huh7 cells, treatment with bavachin (20 μM) significantly suppressed PA/OA or high glucose/high insulin-induced increases in the expression of fatty acid synthesis-related genes and the number and size of lipid droplets. Furthermore, bavachin treatment markedly elevated the phosphorylation levels of AKT and GSK-3β, improving the insulin signaling activity in the cells. In HFD-induced obese mice, administration of bavachin (30 mg/kg, i.p. every other day for 8 weeks) efficiently attenuated the increases in body weight, liver weight, blood glucose, and liver and serum triglyceride contents. Moreover, bavachin administration significantly alleviated hepatic inflammation and ameliorated HFD-induced glucose intolerance and insulin resistance. We demonstrated that bavachin protected against HFD-induced obesity by inducing fat thermogenesis and browning subcutaneous white adipose tissue (subWAT). We revealed that bavachin repressed the expression of lipid synthesis genes in the liver of obese mice, while promoting the expression of thermogenesis, browning, and mitochondrial respiration-related genes in subWAT and brown adipose tissue (BAT) in the mice. In conclusion, bavachin attenuates hepatic steatosis and obesity by repressing de novo lipogenesis, inducing fat thermogenesis and browning subWAT, suggesting that bavachin is a potential drug for NAFLD therapy.
© 2023. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
-
Pharmacology
In Journal of Lipid Research on 1 October 2022 by Ostlund, C., Hernandez-Ono, A., et al.
Lipid droplets (LDs) are generally considered to be synthesized in the ER and utilized in the cytoplasm. However, LDs have been observed inside nuclei in some cells, although recent research on nuclear LDs has focused on cultured cell lines. To better understand nuclear LDs that occur in vivo, here we examined LDs in primary hepatocytes from mice following depletion of the nuclear envelope protein lamina-associated polypeptide 1 (LAP1). Microscopic image analysis showed that LAP1-depleted hepatocytes contain frequent nuclear LDs, which differ from cytoplasmic LDs in their associated proteins. We found type 1 nucleoplasmic reticula, which are invaginations of the inner nuclear membrane, are often associated with nuclear LDs in these hepatocytes. Furthermore, in vivo depletion of the nuclear envelope proteins lamin A and C from mouse hepatocytes led to severely abnormal nuclear morphology, but significantly fewer nuclear LDs than were observed upon depletion of LAP1. In addition, we show both high-fat diet feeding and fasting of mice increased cytoplasmic lipids in LAP1-depleted hepatocytes but reduced nuclear LDs, demonstrating a relationship of LD formation with nutritional state. Finally, depletion of microsomal triglyceride transfer protein did not change the frequency of nuclear LDs in LAP1-depleted hepatocytes, suggesting that it is not required for the biogenesis of nuclear LDs in these cells. Together, these data show that LAP1-depleted hepatocytes represent an ideal mammalian system to investigate the biogenesis of nuclear LDs and their partitioning between the nucleus and cytoplasm in response to changes in nutritional state and cellular metabolism in vivo.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
-
WB
-
Mus musculus (House mouse)
Preprint on BioRxiv : the Preprint Server for Biology on 28 June 2022 by Ostlund, C., Hernandez-Ono, A., et al.
h4>ABSTRACT/h4> Lipid droplets (LDs) are generally considered to be synthesized in the ER and utilized in the cytoplasm. However, LDs have been observed inside nuclei in some cells, although recent research on nuclear LDs has focused on cultured cell lines. To better understand nuclear LDs that occur in vivo , here we examined LDs in primary hepatocytes from mice following depletion of the nuclear envelope protein lamina-associated polypeptide 1 (LAP1). Microscopic image analysis showed that LAP1-depleted hepatocytes contain frequent nuclear LDs, which differ from cytoplasmic LDs in their associated proteins. We found type 1 nucleoplasmic reticula, which are invaginations of the inner nuclear membrane, are often associated with nuclear LDs in these hepatocytes. Furthermore, in vivo depletion of the nuclear envelope proteins lamin A and C from mouse hepatocytes led to severely abnormal nuclear morphology, but significantly fewer nuclear LDs than were observed upon depletion of LAP1. In addition, we show both high fat diet feeding and fasting of mice increased cytoplasmic lipids in LAP1-depleted hepatocytes, but reduced nuclear LDs, demonstrating a relationship of LD formation with nutritional state. Finally, depletion of microsomal triglyceride transfer protein did not change the frequency of nuclear LDs in LAP1-depleted hepatocytes, suggesting that it is not necessary for the biogenesis of nuclear LDs in these cells. Together, these data show that LAP1-depleted hepatocytes represent an ideal mammalian system to investigate the biogenesis of nuclear LDs and their partitioning between the nucleus and cytoplasm in response to changes in nutritional state and cellular metabolism in vivo .
-
Mus musculus (House mouse)
The GR-gp78 Pathway is involved in Hepatic Lipid Accumulation Induced by Overexpression of 11β-HSD1.
In International Journal of Biological Sciences on 1 June 2022 by Hu, M., Han, T., et al.
Glucocorticoids are essential participants in the regulation of lipid metabolism. On a tissue-specific level, glucocorticoid signal is controlled by 11β-Hydroxysteroid dehydrogenase 1 (11β-HSD1). Up-regulation of 11β-HSD1 expression during non-alcoholic fatty liver disease (NAFLD) has been previously shown, while 11β-HSD1 inhibition has been shown to reduce hepatic lipids in NAFLD, but the underlying mechanisms remain unclear. Here, in this study, we created in vitro cell culture and in vivo transgenic hepatocyte-specific 11β-HSD1 mouse models of NAFLD to determine the regulatory mechanisms of 11β-HSD1 during lipid metabolism dysfunction. We found that 11β-HSD1 overexpression activated glucocorticoid receptors and promoted their nuclear translocation, and then stimulating gp78. The induction of gp78 sharply reduced expression of Insig2, but not Insig1, which led to up-regulation of lipogenesis regulatory proteins including SREBP1, FAS, SCD1, and ACC1. Our results suggested that overexpression of 11β-HSD1 induced lipid accumulation, at least partially through the GR/gp78/Insig2/SREBP1 pathway, which may serve as a potential diagnostic and therapeutic target for treatment of NAFLD.
© The author(s).
In Theranostics on 13 October 2020 by Yang, M., Liu, Q., et al.
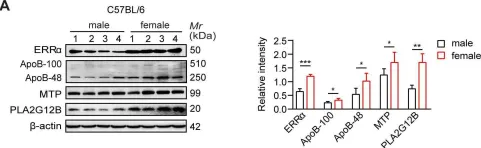
Fig.1.A

-
WB
-
Collected and cropped from Theranostics by CiteAb, provided under a CC-BY license
Image 1 of 7
In Theranostics on 13 October 2020 by Yang, M., Liu, Q., et al.
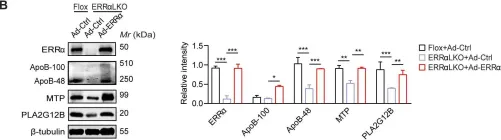
Fig.3.B

-
WB
-
Collected and cropped from Theranostics by CiteAb, provided under a CC-BY license
Image 1 of 7
In Theranostics on 13 October 2020 by Yang, M., Liu, Q., et al.
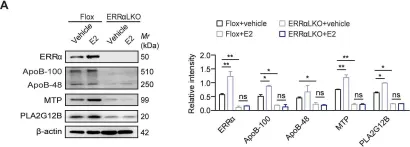
Fig.4.A

-
WB
-
Collected and cropped from Theranostics by CiteAb, provided under a CC-BY license
Image 1 of 7
In Theranostics on 13 October 2020 by Yang, M., Liu, Q., et al.
Fig.6.E

-
WB
-
Collected and cropped from Theranostics by CiteAb, provided under a CC-BY license
Image 1 of 7
In Theranostics on 13 October 2020 by Yang, M., Liu, Q., et al.
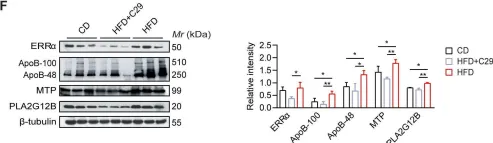
Fig.7.F

-
WB
-
Collected and cropped from Theranostics by CiteAb, provided under a CC-BY license
Image 1 of 7
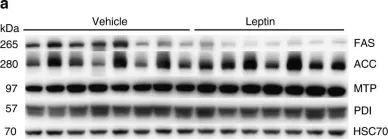
In Nat Commun on 20 June 2019 by Hackl, M. T., Fürnsinn, C., et al.
Fig.3.A

-
WB
-
Rattus norvegicus (Rat)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 7
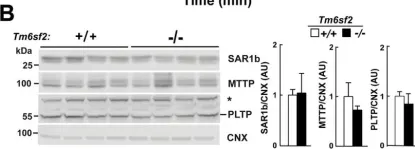
In J Biol Chem on 13 May 2016 by Smagris, E., Gilyard, S., et al.
Fig.11.B

-
WB
-
Collected and cropped from J Biol Chem by CiteAb, provided under a CC-BY license
Image 1 of 7