The immune response is considered a significant pathological mechanism of inner ear damage. However, the role of macrophages, as key components of immune cells, in immunity in the inner ear remains elusive. Evidence from other organs indicates that phagocytosis, a core function of macrophages, plays a crucial role in maintaining homeostasis, development, and tissue repair regeneration. However, it has rarely been studied in the inner ear. This field may currently hold new insights. In this study, we aimed to investigate the immunological contribution of resident macrophages in the inner ear to cisplatin-induced ototoxicity. By using clodronate liposomes and cytochalasin to deplete macrophages or inhibit macrophage phagocytosis locally, we first elucidated the dynamic changes in the immune state of inner ear macrophages during cisplatin injury through multimodal and multidimensional approaches. High-spatiotemporal-resolution single-cell analysis and real-time imaging of macrophages during zebrafish hair cell death identified proinflammatory subsets during cisplatin injury. We found that macrophage activation through phagocytosis synergized with the inflammatory response and that inhibiting macrophage phagocytosis could ameliorate cisplatin-induced ototoxicity. Finally, we discuss how the highly plastic phagocytic function of resident macrophages in the inner ear holds potential for the development of strategies for treating cisplatin-induced hearing loss.
© 2025. The Author(s).
Product Citations: 526
Inhibition of inner ear macrophage phagocytosis alleviates cisplatin-induced ototoxicity.
In Communications Biology on 30 July 2025 by Zhang, J., Zhang, W., et al.
-
Immunology and Microbiology
In Neuroscience Bulletin on 20 July 2025 by Ma, Q., Wang, Q., et al.
Circadian sensitivity significantly influences the severity of noise-induced hearing loss (NIHL), but the underlying mechanisms remain unclear. Here, we applied single-cell RNA sequencing to 97,043 cochlear cells, identifying macrophages as the primary immune responders to acoustic trauma, with a notable increase in their proportion in the cochlea. Immunofluorescence confirmed significant recruitment and activation of cochlear macrophages following noise exposure, while in vivo macrophage depletion resulted in the recovery of hearing. Furthermore, analyses of differentially-expressed genes and pathways revealed pronounced activation of NLRP3 inflammasome signaling in macrophages during night-time noise exposure. Measurements of elevated IL-1β and IL-18 expression in cochlear macrophages by multiplex immunohistochemistry correlated with heightened inflammation in the night-time exposure group. These findings were further confirmed by the administration of the selective NLRP3 inhibitor CY-09, which mitigated inflammasome activation, preserved synaptic integrity, and protect against hearing loss. In conclusion, our findings underscore the role of macrophage-driven NLRP3 inflammasome activation in mediating circadian variations in cochlear damage, offering a potential therapeutic target for mitigating NIHL.
© 2025. The Author(s).
-
Immunology and Microbiology
In Scientific Reports on 15 July 2025 by Lee, H. M., Kim, J., et al.
Alzheimer's disease (AD) is an age-related neurodegenerative disorder characterized by neuronal and synaptic loss in the brain, which leads to cognitive impairment and dementia. Therefore, early diagnosis by employing various biomarkers is crucial for preventing and treating AD. Although retinal pathology is an emerging biomarker associated with AD, detailed molecular mechanisms of retinal impairments remain unclear. Herein, we identified genome-wide dysfunction of alternative splicing in the early stage of 5xFAD transgenic mouse retina by performing RNA sequencing analysis. Notably, retained introns, highly enriched in phototransduction and retinal genes in the 1.5-month-old 5xFAD mouse retina, was significantly associated with physiological impairment of rod photoreceptors in the retina, as evidenced by electroretinogram (ERG) analysis. These results indicate that the abnormal scotopic ERG associated with global splicing impairment may be valuable as an early-detection biomarker for AD.
© 2025. The Author(s).
-
Neuroscience
Gating of hair cell Ca2+ channels governs the activity of cochlear neurons.
In Science Advances on 20 June 2025 by Karagulyan, N., Thirumalai, A., et al.
Our sense of hearing processes sound intensities spanning six orders of magnitude. In the ear, the receptor potential of presynaptic inner hair cells (IHCs) covers the entire intensity range, while postsynaptic spiral ganglion neurons (SGNs) tile the range with their firing rate codes. IHCs vary the voltage dependence of Ca2+ channel activation among their active zones (AZs), potentially diversifying SGN firing. Here, we tested this hypothesis in mice modeling the human CaV1.3A749G mutation that causes low-voltage Ca2+ channel activation. We demonstrate activation of Ca2+ influx and glutamate release of IHC AZs at lower voltages, increased spontaneous firing in SGNs, and lower sound threshold of CaV1.3A749G/A749G mice. Loss of synaptic ribbons in IHCs at ambient sound levels of mouse husbandry indicates that low-voltage Ca2+ channel activation poses a risk for noise-induced synaptic damage. We propose that the heterogeneous voltage dependence of CaV1.3 activation among presynaptic IHC AZs contributes to the diversity of firing among the postsynaptic SGNs.
-
Neuroscience
In Molecular Therapy. Methods Clinical Development on 12 June 2025 by Slika, E., Fuchs, P. A., et al.
Noise-induced hearing loss (NIHL) poses an emerging global health problem with only ear protection or sound avoidance as preventive strategies. The cochlea receives some protection from medial olivocochlear efferent neurons, providing a potential target for therapeutic enhancement. Cholinergic efferents release acetylcholine (ACh) to hyperpolarize and shunt the outer hair cells (OHCs), reducing sound-evoked activation. The (α9)2(α10)3 nicotinic ACh receptor (nAChR) on the OHCs mediates this effect. Transgenic knockin mice with a gain-of-function nAChR (α9L9'T) suffer less NIHL. α9 knockout mice are more vulnerable to NIHL but can be rescued by viral transduction of the α9L9'T subunit. In this study, an HA-tagged gain-of-function α9 isoform was expressed in wild-type mice to reduce NIHL. Synaptic integration of the virally expressed nAChR subunit was confirmed by HA immunopuncta localized to the postsynaptic membrane of OHCs. After noise exposure, AAV2.7m8-CAG-α9L9'T-HA (α9L9'T-HA)-injected mice had less hearing loss (auditory brainstem response [ABR] thresholds and threshold shifts) than did control mice. ABRs of α9L9'T-HA-injected mice also had larger wave-1 amplitudes and better recovery of wave-1 amplitudes post noise exposure. Thus, virally expressed α9L9'T combines effectively with native α9 and α10 subunits to mitigate NIHL in wild-type cochleas.
© 2025 The Authors.
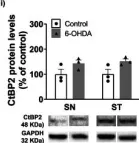
In Mol Neurobiol on 1 August 2023 by Saraiva, C., Lopes-Nunes, J., et al.
Fig.4.I

-
WB
-
Collected and cropped from Mol Neurobiol by CiteAb, provided under a CC-BY license
Image 1 of 21
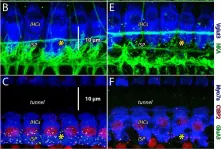
In Front Synaptic Neurosci on 23 July 2021 by Walia, A., Lee, C., et al.
Fig.3.B

-
IHC-IF
-
Collected and cropped from Front Synaptic Neurosci by CiteAb, provided under a CC-BY license
Image 1 of 21
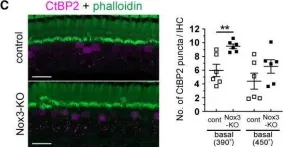
In J Neurosci on 26 May 2021 by Mohri, H., Ninoyu, Y., et al.
Fig.4.C

-
IHC-IF
-
Mus musculus (House mouse)
Collected and cropped from J Neurosci by CiteAb, provided under a CC-BY license
Image 1 of 21
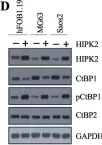
In J Cancer on 23 February 2021 by Duan, N., Zhang, W., et al.
Fig.2.D

-
WB
-
Collected and cropped from J Cancer by CiteAb, provided under a CC-BY license
Image 1 of 21
In Int J Biol Sci on 7 March 2020 by Sun, X., Xiao, L., et al.
Fig.1.C

-
WB
-
Collected and cropped from Int J Biol Sci by CiteAb, provided under a CC-BY license
Image 1 of 21
In Int J Biol Sci on 7 March 2020 by Sun, X., Xiao, L., et al.
Fig.3.B

-
WB
-
Collected and cropped from Int J Biol Sci by CiteAb, provided under a CC-BY license
Image 1 of 21
In Int J Biol Sci on 7 March 2020 by Sun, X., Xiao, L., et al.
Fig.3.C

-
WB
-
Collected and cropped from Int J Biol Sci by CiteAb, provided under a CC-BY license
Image 1 of 21
In Int J Biol Sci on 7 March 2020 by Sun, X., Xiao, L., et al.
Fig.5.A

-
WB
-
Collected and cropped from Int J Biol Sci by CiteAb, provided under a CC-BY license
Image 1 of 21
In Int J Biol Sci on 23 November 2019 by Chen, Z., Dong, W. H., et al.
Fig.7.B

-
WB
-
Homo sapiens (Human)
Collected and cropped from Int J Biol Sci by CiteAb, provided under a CC-BY license
Image 1 of 21
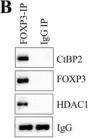
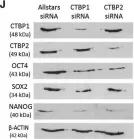
In Stem Cell Reports on 9 April 2019 by Arthur, S. A., Blaydes, J. P., et al.
Fig.5.A

-
WB
-
Collected and cropped from Stem Cell Reports by CiteAb, provided under a CC-BY license
Image 1 of 21
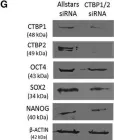
In Stem Cell Reports on 9 April 2019 by Arthur, S. A., Blaydes, J. P., et al.
Fig.4.G

-
WB
-
Collected and cropped from Stem Cell Reports by CiteAb, provided under a CC-BY license
Image 1 of 21
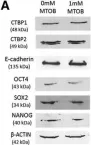
In Stem Cell Reports on 9 April 2019 by Arthur, S. A., Blaydes, J. P., et al.
Fig.4.D

-
WB
-
Collected and cropped from Stem Cell Reports by CiteAb, provided under a CC-BY license
Image 1 of 21
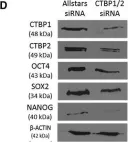
In Stem Cell Reports on 9 April 2019 by Arthur, S. A., Blaydes, J. P., et al.
Fig.4.J

-
WB
-
Collected and cropped from Stem Cell Reports by CiteAb, provided under a CC-BY license
Image 1 of 21
In Stem Cell Reports on 9 April 2019 by Arthur, S. A., Blaydes, J. P., et al.
Fig.1.G

-
ICC-IF
-
Collected and cropped from Stem Cell Reports by CiteAb, provided under a CC-BY license
Image 1 of 21
In Stem Cell Reports on 9 April 2019 by Arthur, S. A., Blaydes, J. P., et al.
Fig.1.B

-
WB
-
Collected and cropped from Stem Cell Reports by CiteAb, provided under a CC-BY license
Image 1 of 21
In Stem Cell Reports on 9 April 2019 by Arthur, S. A., Blaydes, J. P., et al.
Fig.1.D

-
WB
-
Collected and cropped from Stem Cell Reports by CiteAb, provided under a CC-BY license
Image 1 of 21
In Stem Cell Reports on 9 April 2019 by Arthur, S. A., Blaydes, J. P., et al.
Fig.1.F

-
ICC-IF
-
Collected and cropped from Stem Cell Reports by CiteAb, provided under a CC-BY license
Image 1 of 21
In Gene Ther on 1 July 2018 by Chen, H., Xing, Y., et al.
Fig.5.A

-
IHC
-
Cavia porcellus (Guinea Pig)
Collected and cropped from Gene Ther by CiteAb, provided under a CC-BY license
Image 1 of 21
In PLoS One on 1 September 2017 by Krebs, M. P., Collin, G. B., et al.
Fig.11.B

-
IHC-IF
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 21
In PLoS One on 7 March 2014 by Storm, M. P., Kumpfmueller, B., et al.
Fig.7.C

-
WB
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 21
In BMC Genomics on 27 December 2007 by Schulz, T. C., Swistowska, A. M., et al.
Fig.4.C

-
ICC-IF
-
Homo sapiens (Human)
Collected and cropped from BMC Genomics by CiteAb, provided under a CC-BY license
Image 1 of 21