Estrogen-dependent breast cancers rely on a constant supply of estrogens and expression of estrogen receptors. Local biosynthesis, by aromatase in breast adipose fibroblasts (BAFs), is their most important source for estrogens. Triple-negative breast cancers (TNBC) rely on other growth-promoting signals, including those from the Wnt pathway. In this study, we explored the hypothesis that Wnt signaling alters the proliferation of BAFs, and is involved in regulation of aromatase expression in BAFs. Conditioned medium (CM) from TNBC cells and WNT3a consistently increased BAF growth, and reduced aromatase activity up to 90%, by suppression of the aromatase promoter I.3/II region. Database searches identified three putative Wnt-responsive elements (WREs) in the aromatase promoter I.3/II. In luciferase reporter gene assays, promoter I.3/II activity was inhibited by overexpression of full-length T-cell factor (TCF)-4 in 3T3-L1 preadipocytes, which served as a model for BAFs. Full-length lymphoid enhancer-binding factor (LEF)-1 increased the transcriptional activity. However, TCF-4 binding to WRE1 in the aromatase promoter, was lost after WNT3a stimulation in immunoprecipitation-based in vitro DNA-binding assays, and in chromatin immunoprecipitation (ChIP). In vitro DNA-binding assays, ChIP, and Western blotting revealed a WNT3a-dependent switch of nuclear LEF-1 isoforms towards a truncated variant, whereas β-catenin levels remained unchanged. This LEF-1 variant revealed dominant negative properties, and most likely recruited enzymes involved in heterochromatin formation. In addition, WNT3a induced the replacement of TCF-4 by the truncated LEF-1 variant, on WRE1 of the aromatase promoter I.3/II. The mechanism described here may be responsible for the loss of aromatase expression predominantly associated with TNBC. Tumors with (strong) expression of Wnt ligands actively suppress aromatase expression in BAFs. Consequently a reduced estrogen supply could favor the growth of estrogen-independent tumor cells, which consequently would make estrogen receptors dispensable. In summary, canonical Wnt signaling within (cancerous) breast tissue may be a major factor controlling local estrogen synthesis and action.
Product Citations: 11
In International Journal of Molecular Sciences on 28 February 2023 by Kaiser, A., Eiselt, G., et al.
-
WB
-
Mus musculus (House mouse)
-
Endocrinology and Physiology
Loss of epigenetic information as a cause of mammalian aging.
In Cell on 19 January 2023 by Yang, J. H., Hayano, M., et al.
All living things experience an increase in entropy, manifested as a loss of genetic and epigenetic information. In yeast, epigenetic information is lost over time due to the relocalization of chromatin-modifying proteins to DNA breaks, causing cells to lose their identity, a hallmark of yeast aging. Using a system called "ICE" (inducible changes to the epigenome), we find that the act of faithful DNA repair advances aging at physiological, cognitive, and molecular levels, including erosion of the epigenetic landscape, cellular exdifferentiation, senescence, and advancement of the DNA methylation clock, which can be reversed by OSK-mediated rejuvenation. These data are consistent with the information theory of aging, which states that a loss of epigenetic information is a reversible cause of aging.
Copyright © 2022 Elsevier Inc. All rights reserved.
-
Genetics
In Science Advances on 10 December 2021 by Clayton, S. A., Daley, K. K., et al.
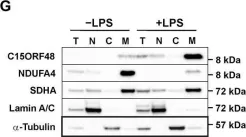
Dysregulated mitochondrial function is a hallmark of immune-mediated inflammatory diseases. Cytochrome c oxidase (CcO), which mediates the rate-limiting step in mitochondrial respiration, is remodeled during development and in response to changes of oxygen availability, but there has been little study of CcO remodeling during inflammation. Here, we describe an elegant molecular switch mediated by the bifunctional transcript C15orf48, which orchestrates the substitution of the CcO subunit NDUFA4 by its paralog C15ORF48 in primary macrophages. Expression of C15orf48 is a conserved response to inflammatory signals and occurs in many immune-related pathologies. In rheumatoid arthritis, C15orf48 mRNA is elevated in peripheral monocytes and proinflammatory synovial tissue macrophages, and its expression positively correlates with disease severity and declines in remission. C15orf48 is also expressed by pathogenic macrophages in severe coronavirus disease 2019 (COVID-19). Study of a rare metabolic disease syndrome provides evidence that loss of the NDUFA4 subunit supports proinflammatory macrophage functions.
-
WB
-
Cell Biology
-
Immunology and Microbiology
In Viruses on 13 July 2021 by Hainley, L. E., Hughson, M. S., et al.
The human BK polyomavirus (BKPyV) is latent in the kidneys of most adults, but can be reactivated in immunosuppressed states, such as following renal transplantation. If left unchecked, BK polyomavirus nephropathy (PyVAN) and possible graft loss may result from viral destruction of tubular epithelial cells and interstitial fibrosis. When coupled with regular post-transplant screening, immunosuppression reduction has been effective in limiting BKPyV viremia and the development of PyVAN. Antiviral drugs that are safe and effective in combating BKPyV have not been identified but would be a benefit in complementing or replacing immunosuppression reduction. The present study explores inhibition of the host DNA damage response (DDR) as an antiviral strategy. Immunohistochemical and immunofluorescent analyses of PyVAN biopsies provide evidence for stimulation of a DDR in vivo. DDR pathways were also stimulated in vitro following BKPyV infection of low-passage human renal proximal tubule epithelial cells. The role of Chk1, a protein kinase known to be involved in the replication stress-induced DDR, was examined by inhibition with the small molecule LY2603618 and by siRNA-mediated knockdown. Inhibition of Chk1 resulted in decreased replication of BKPyV DNA and viral spread. Activation of mitotic pathways was associated with the reduction in BKPyV replication. Chk1 inhibitors that are found to be safe and effective in clinical trials for cancer should also be evaluated for antiviral activity against BKPyV.
-
WB
-
Genetics
-
Immunology and Microbiology
In Oncology Letters on 1 March 2019 by Park, S. Y., Kim, D., et al.
Metformin can suppress cell proliferation and viability by altering mitochondrial energy metabolism and by the activation of 5'-adenosine monophosphate-activated protein kinase (AMPK). The current study demonstrated that metformin-induced suppression of cell proliferation is further potentiated by AMPK-mediated suppression of β-catenin-dependent wingless-type (Wnt) signaling. Treatment with metformin reduced mitochondrial oxidative phosphorylation and glycolysis, leading to an energy imbalance that may induce AMPK phosphorylation in RKO cells. Metformin treatment also decreased β-catenin expression in the cytoplasm and nucleus. Active AMPK was revealed to be associated with β-catenin. The decrease in β-catenin expression was inhibited by proteosome inhibition through phosphorylation of β-catenin at serine 33/37. Given that nuclear translocation-associated phosphorylation of β-catenin at serine was maintained, the association of β-catenin with AMPK may sequester β-catenin in the cytoplasm and lead to proteosomal degradation. Furthermore, metformin-induced suppression of cell proliferation was partially recovered by AMPK inhibition, while metformin inhibited Wnt-mediated cell proliferation and β-catenin expression. The present results suggest that AMPK activation can suppress β-catenin-dependent Wnt signaling by cytoplasmic sequestering of β-catenin through AMPK, which further decreases cell proliferation in addition to metformin-induced mitochondrial dysfunction.
-
WB
-
Homo sapiens (Human)
-
Cancer Research
In Sci Adv on 10 December 2021 by Clayton, S. A., Daley, K. K., et al.
Fig.3.G

-
WB
-
Collected and cropped from Sci Adv by CiteAb, provided under a CC-BY license
Image 1 of 1