Endurance exercise is associated with an increased risk of atrial fibrillation (AF). We previously established that adverse atrial remodelling and AF susceptibility induced by intense exercise in mice require the mechanosensitive and pro-inflammatory cytokine tumour necrosis factor (TNF). The cellular and mechanistic basis for these TNF-mediated effects is unknown.
We studied the impact of Tnf excision, in either atrial cardiomyocytes or endothelial cells (using Cre-recombinase expression controlled by Nppa or Tie2 promoters, respectively), on the cardiac responses to six weeks of intense swim exercise training. TNF ablation, in either cell type, had no impact on the changes in heart rate, autonomic tone, or left ventricular structure and function induced by exercise training. Tnf excision in atrial cardiomyocytes did, however, prevent atrial hypertrophy, fibrosis, and macrophage infiltration as well as conduction slowing and increased AF susceptibility arising from exercise training. In contrast, endothelial-specific excision only reduced the training-induced atrial hypertrophy. Consistent with these cell-specific effects of Tnf excision, inducing TNF loss from atrial cardiomyocytes prevented activation of p38MAPKinase, a strain-dependent downstream mediator of TNF signalling, without affecting the atrial stretch as assessed by atrial pressures induced by exercise. Despite TNF's established role in innate immune responses and inflammation, neither acute nor chronic exercise training caused measurable NLRP3 inflammasome activation.
Our findings demonstrate that adverse atrial remodelling and AF vulnerability induced by intense exercise require TNF in atrial cardiomyocytes whereas the impact of endothelial-derived TNF is limited to hypertrophy modulation. The implications of the cell autonomous effects of TNF and crosstalk between cells in the atria are discussed.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Product Citations: 16
In Cardiovascular Research on 19 December 2023 by Lakin, R., Polidovitch, N., et al.
-
Mus musculus (House mouse)
-
Cancer Research
-
Cardiovascular biology
In Environmental Toxicology on 1 February 2023 by Lee, C. Y., Ho, Y. C., et al.
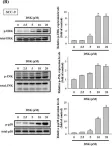
Diphenyl difluoroketone (EF-24), a synthetic curcumin analog, has enhanced bioavailability over curcumin. EF-24 acts more powerful bioactivity for anti-inflammatory and anti-cancer activity. However, the effects and mechanism of EF-24 on cervical cancer has not been fully investigated. Herein, this study evaluated the effects of EF-24 on TPA-induced cellular migration of cervical cancer. The results showed that EF-24 substantially reduced the cellular migration and cellular invasion of the HeLa and SiHa cells. Moreover, gelatin zymography, western blotting analyses and real-time PCR revealed that EF-24 suppressed Matrix metalloproteinase-9 (MMP-9) activity, protein expression and mRNA levels. Mechanistically, EF-24 inhibited the phosphorylation of the p38 signaling pathway. In conclusion, EF-24 inhibited TPA-induced cellular migration and cellular invasion of cervical cancer cell lines through modulating MMP-9 expression via downregulating signaling p38 pathway and EF-24 may have potential to serve as a chemopreventive agent of cervical cancer.
© 2022 Wiley Periodicals LLC.
-
WB
-
Homo sapiens (Human)
-
Cancer Research
In International Journal of Molecular Sciences on 26 June 2022 by Chuang, C. Y., Lin, C. W., et al.
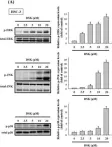
Deoxyshikonin (DSK), a phytochemical constituent, has been documented to elicit various oncostatic properties alone or in combination with established therapeutics. However, its role in restraining oral squamous cell carcinoma (OSCC) is mostly unclear. Here, we examined the tumor-suppressive effect of DSK and explored the molecular mechanisms underlying DSK's activities on controlling oral cancer. Our results showed that DSK dose-dependently lessened the cell viability of tongue cancer cell lines, involving induction of cell cycle arrest at the sub-G1 phase and apoptotic cell death. Moreover, a unique signature of apoptosis-related proteins, including augmented nuclear factor erythroid 2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) expression and caspase activation, was observed in DSK-treated tongue cancer cell lines. Furthermore, DSK-mediated upregulation of HO-1 and cleavage of caspase-9 and -3 were significantly inhibited by pharmacological blockage of p38 kinase. Collectively, these data revealed that DSK halted cell cycle progression and elicited cell apoptosis in tongue cancer cell lines, reshaping a p38-dependent profile of apoptotic proteome. Our findings provided novel insights into the therapeutic implications of a natural compound on the management of OSCC.
-
WB
-
Cancer Research
In Oncology Reports on 1 March 2022 by Kadota, A., Moriguchi, M., et al.
Primary effusion lymphoma (PEL) is defined as a rare subtype of non‑Hodgkin's B cell lymphoma, which is caused by Kaposi's sarcoma‑associated herpesvirus (KSHV) in immunosuppressed patients. PEL is an aggressive type of lymphoma and is frequently resistant to conventional chemotherapeutics. Therefore, the discovery of novel drug candidates for the treatment of PEL is of utmost importance. In order to discover potential novel anti‑tumor compounds against PEL, the authors previously developed a pyrrolidinium‑type fullerene derivative, 1,1,1',1'‑tetramethyl [60]fullerenodipyrrolidinium diiodide (derivative #1), which induced the apoptosis of PEL cells via caspase‑9 activation. In the present study, the growth inhibitory effects of pyrrolidinium‑type (derivatives #1 and #2), pyridinium‑type (derivatives #3 and #5 to #9) and anilinium‑type fullerene derivatives (derivative #4) against PEL cells were evaluated. This analysis revealed a pyridinium‑type derivative (derivative #5; 3‑5'‑(etho xycarbonyl)‑1',5'‑dihydro‑2'H‑[5,6]fullereno‑C60‑Ih‑[1,9‑c]pyrrol‑2'‑yl]‑1‑methylpyridinium iodide), which exhibited antitumor activity against PEL cells via the downregulation of Wnt/β‑catenin signaling. Derivative #5 suppressed the viability of KSHV‑infected PEL cells compared with KSHV‑uninfected B‑lymphoma cells. Furthermore, derivative #5 induced the destabilization of β‑catenin and suppressed β‑catenin‑TCF4 transcriptional activity in PEL cells. It is known that the constitutive activation of Wnt/β‑catenin signaling is essential for the growth of KSHV‑infected cells. The Wnt/β‑catenin activation in KSHV‑infected cells is mediated by KSHV latency‑associated nuclear antigen (LANA). The data demonstrated that derivative #5 increased β‑catenin phosphorylation, which resulted in β‑catenin polyubiquitination and subsequent degradation. Thus, derivative #5 overcame LANA‑mediated β‑catenin stabilization. Furthermore, the administration of derivative #5 suppressed the development of PEL cells in the ascites of SCID mice with tumor xenografts derived from PEL cells. On the whole, these findings provide evidence that the pyridinium‑type fullerene derivative #5 exhibits antitumor activity against PEL cells in vitro and in vivo. Thus, derivative #5 may be utilized as a novel therapeutic agent for the treatment of PEL.
-
Cancer Research
In International Journal of Molecular Sciences on 1 January 2022 by Yeh, L. T., Lin, C. W., et al.
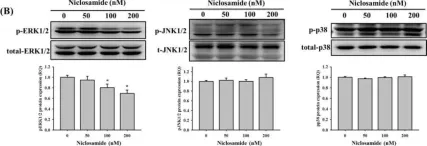
Osteosarcoma is a highly common malignant bone tumor. Its highly metastatic properties are the leading cause of mortality for cancer. Niclosamide, a salicylanilide derivative, is an oral antihelminthic drug of known anticancer potential. However, the effect of niclosamide on osteosarcoma cell migration, invasion and the mechanisms underlying have not been fully clarified. Therefore, this study investigated niclosamide's underlying pathways and antimetastatic effects on osteosarcoma. In this study, U2OS and HOS osteosarcoma cell lines were treated with niclosamide and then subjected to assays for determining cell migration ability. The results indicated that niclosamide, at concentrations of up to 200 nM, inhibited the migration and invasion of human osteosarcoma U2OS and HOS cells and repressed the transforming growth factor beta-induced protein (TGFBI) expression of U2OS cells, without cytotoxicity. After TGFBI knockdown occurred, cellular migration and invasion behaviors of U2OS cells were significantly reduced. Moreover, niclosamide significantly decreased the phosphorylation of ERK1/2 in U2OS cells and the combination treatment of the MEK inhibitor (U0126) and niclosamide resulted in the intensive inhibition of the TGFBI expression and the migratory ability in U2OS cells. Therefore, TGFBI derived from osteosarcoma cells via the ERK pathway contributed to cellular migration and invasion and niclosamide inhibited these processes. These findings indicate that niclosamide may be a powerful preventive agent against the development and metastasis of osteosarcoma.
-
WB
-
Homo sapiens (Human)
-
Cancer Research
In Int J Mol Sci on 26 June 2022 by Chuang, C. Y., Lin, C. W., et al.
Fig.6.A

-
WB
-
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 3
In Int J Mol Sci on 26 June 2022 by Chuang, C. Y., Lin, C. W., et al.
Fig.6.B

-
WB
-
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 3
In Int J Mol Sci on 1 January 2022 by Yeh, L. T., Lin, C. W., et al.
Fig.5.B

-
WB
-
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 3