Kainate receptors (KARs) are one of the ionotropic glutamate receptors in the central nervous system (CNS) comprised of five subunits, GluK1-GluK5. There is a growing interest in the association between KARs and psychiatric disorders, and there have been several studies investigating the behavioral phenotypes of KAR deficient mice, however, the difference in the genetic background has been found to affect phenotype in multiple mouse models of human diseases. Here, we examined GluK1-5 single KO mice in a pure C57BL/6N background and identified that GluK3 KO mice specifically express anxiolytic-like behavior with an alteration in dopamine D2 receptor (D2R)-induced anxiety, and reduced D2R expression in the striatum. Biochemical studies in the mouse cortex confirmed that GluK3 subunits do not assemble with GluK4 and GluK5 subunits, that can be activated by lower concentration of agonists. Overall, we found that GluK3-containing KARs function to express anxiety, which may represent promising anti-anxiety medication targets.
© 2024. The Author(s).
Product Citations: 9
Behavioral analysis of kainate receptor KO mice and the role of GluK3 subunit in anxiety.
In Scientific Reports on 24 February 2024 by Iida, I., Konno, K., et al.
-
Mus musculus (House mouse)
In Experimental & Molecular Medicine on 1 August 2022 by Bae, Y. S., Yoon, S. H., et al.
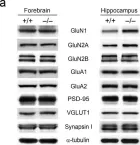
Inborn errors of metabolism (IEMs) are common causes of neurodevelopmental disorders, including microcephaly, hyperactivity, and intellectual disability. However, the synaptic mechanisms of and pharmacological interventions for the neurological complications of most IEMs are unclear. Here, we report that metabolic dysfunction perturbs neuronal NMDA receptor (NMDAR) homeostasis and that the restoration of NMDAR signaling ameliorates neurodevelopmental and cognitive deficits in IEM model mice that lack aminopeptidase P1. Aminopeptidase P1-deficient (Xpnpep1-/-) mice, with a disruption of the proline-specific metalloprotease gene Xpnpep1, exhibit hippocampal neurodegeneration, behavioral hyperactivity, and impaired hippocampus-dependent learning. In this study, we found that GluN1 and GluN2A expression, NMDAR activity, and the NMDAR-dependent long-term potentiation (LTP) of excitatory synaptic transmission were markedly enhanced in the hippocampi of Xpnpep1-/- mice. The exaggerated NMDAR activity and NMDAR-dependent LTP were reversed by the NMDAR antagonist memantine. A single administration of memantine reversed hyperactivity in adult Xpnpep1-/- mice without improving learning and memory. Furthermore, chronic administration of memantine ameliorated hippocampal neurodegeneration, hyperactivity, and impaired learning and memory in Xpnpep1-/- mice. In addition, abnormally enhanced NMDAR-dependent LTP and NMDAR downstream signaling in the hippocampi of Xpnpep1-/- mice were reversed by chronic memantine treatment. These results suggest that the metabolic dysfunction caused by aminopeptidase P1 deficiency leads to synaptic dysfunction with excessive NMDAR activity, and the restoration of synaptic function may be a potential therapeutic strategy for the treatment of neurological complications related to IEMs.
© 2022. The Author(s).
-
WB
-
Mus musculus (House mouse)
-
Biochemistry and Molecular biology
GABA interneurons are the cellular trigger for ketamine's rapid antidepressant actions.
In The Journal of Clinical Investigation on 2 March 2020 by Gerhard, D. M., Pothula, S., et al.
A single subanesthetic dose of ketamine, an NMDA receptor (NMDAR) antagonist, produces rapid and sustained antidepressant actions in depressed patients, addressing a major unmet need for the treatment of mood disorders. Ketamine produces a rapid increase in extracellular glutamate and synaptic formation in the prefrontal cortex, but the initial cellular trigger that initiates this increase and ketamine's behavioral actions has not been identified. To address this question, we used a combination of viral shRNA and conditional mutation to produce cell-specific knockdown or deletion of a key NMDAR subunit, GluN2B, implicated in the actions of ketamine. The results demonstrated that the antidepressant actions of ketamine were blocked by GluN2B-NMDAR knockdown on GABA (Gad1) interneurons, as well as subtypes expressing somatostatin (Sst) or parvalbumin (Pvalb), but not glutamate principle neurons in the medial prefrontal cortex (mPFC). Further analysis of GABA subtypes showed that cell-specific knockdown or deletion of GluN2B in Sst interneurons blocked or occluded the antidepressant actions of ketamine and revealed sex-specific differences that are associated with excitatory postsynaptic currents on mPFC principle neurons. These findings demonstrate that GluN2B-NMDARs on GABA interneurons are the initial cellular trigger for the rapid antidepressant actions of ketamine and show sex-specific adaptive mechanisms to GluN2B modulation.
In Current Biology : CB on 22 July 2019 by Pilarzyk, K., Klett, J., et al.
Systems consolidation is a process by which memories initially require the hippocampus for recent long-term memory (LTM) but then become increasingly independent of the hippocampus and more dependent on the cortex for remote LTM. Here, we study the role of phosphodiesterase 11A4 (PDE11A4) in systems consolidation. PDE11A4, which degrades cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP), is preferentially expressed in neurons of CA1, the subiculum, and the adjacently connected amygdalohippocampal region. In male and female mice, deletion of PDE11A enhances remote LTM for social odor recognition and social transmission of food preference (STFP) despite eliminating or silencing recent LTM for those same social events. Measurement of a surrogate marker of neuronal activation (i.e., Arc mRNA) suggests the recent LTM deficits observed in Pde11 knockout mice correspond with decreased activation of ventral CA1 relative to wild-type littermates. In contrast, the enhanced remote LTM observed in Pde11a knockout mice corresponds with increased activation and altered functional connectivity of anterior cingulate cortex, frontal association cortex, parasubiculum, and the superficial layer of medial entorhinal cortex. The apparent increased neural activation observed in prefrontal cortex of Pde11a knockout mice during remote LTM retrieval may be related to an upregulation of the N-methyl-D-aspartate receptor subunits NR1 and NR2A. Viral restoration of PDE11A4 to vCA1 alone is sufficient to rescue both the LTM phenotypes and upregulation of NR1 exhibited by Pde11a knockout mice. Together, our findings suggest remote LTM can be decoupled from recent LTM, which may have relevance for cognitive deficits associated with aging, temporal lobe epilepsy, or transient global amnesia.
Copyright © 2019 Elsevier Ltd. All rights reserved.
-
WB
In PLoS ONE on 21 December 2017 by Elnagar, M. R., Walls, A. B., et al.
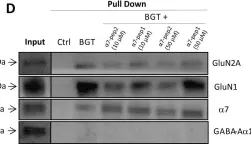
α7 nicotinic acetylcholine receptors (nAChRs) and N-methyl-D-aspartate receptors (NMDARs) are key mediators of central cholinergic and glutamatergic neurotransmission, respectively. In addition to numerous well-established functional interactions between α7 nAChRs and NMDARs, the two receptors have been proposed to form a multimeric complex, and in the present study we have investigated this putative α7 nAChR/NMDAR assembly in human and murine brain tissues. By α-bungarotoxin (BGT) affinity purification, α7 and NMDAR subunits were co-purified from human and murine cortical and hippocampal homogenates, substantiating the notion that the receptors are parts of a multimeric complex in the human and rodent brain. Interestingly, the ratios between GluN1 and α7 levels in BGT pull-downs from cortical homogenates from Alzheimer's disease (AD) brains were significantly lower than those in pull-downs from non-AD controls, indicating a reduced degree of α7 nAChR/NMDAR complex formation in the diseased tissue. A similar difference in GluN1/α7 ratios was observed between pull-downs from cortical homogenates from adult 3xTg-AD and age-matched wild type (WT) mice, whereas the GluN1/α7 ratios determined in pull-downs from young 3xTg-AD and age-matched WT mice did not differ significantly. The observation that pretreatment with oligomeric amyloid-β1-42 reduced GluN1/α7 ratios in BGT pull-downs from human cortical homogenate in a concentration-dependent manner provided a plausible molecular mechanism for this observed reduction. In conclusion, while it will be important to further challenge the existence of the putative α7 nAChR/NMDAR complex in future studies applying other methodologies than biochemical assays and to investigate the functional implications of this complex for cholinergic and glutamatergic neurotransmission, this work supports the formation of the complex and presents new insights into its regulation in healthy and diseased brain tissue.
-
WB
-
Neuroscience
In Exp Mol Med on 1 August 2022 by Bae, Y. S., Yoon, S. H., et al.
Fig.1.A

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Exp Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 2
In PLoS One on 21 December 2017 by Elnagar, M. R., Walls, A. B., et al.
Fig.1.D

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 2