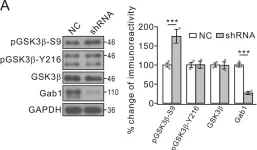
In approximately 80% of colorectal cancer cases, mutations in the adenomatous polyposis coli (APC) gene disrupt the Wingless-related integration site (Wnt)/β-catenin signaling pathway, a crucial factor in carcinogenesis. This disruption may result in consequences such as aberrant spindle segregation and mitotic catastrophe. This study aimed to analyze the effectiveness of the ethanolic extract of red okra (Abelmoschus esculentus) pods (EEROP) in inducing apoptosis in colorectal cancer cells (SW480) by inhibiting the Wnt/β-catenin signaling pathway.
The IC50 of EEROP in SW480 cells was determined by treating the cells with varying doses of EEROP, ranging from 0 to 1000 µg/mL. Apoptosis assay and signaling pathway analysis were performed through immunofluorescence staining and Western Blotting on SW480 cells treated with 250 µg/mL of EEROP for 72 hours.
EEROP treatment induced apoptosis in SW480 cells, marked by elevated levels of active caspase-3 (P<0.001) and cleaved poly-(ADP-ribose) polymerase (PARP)-1. Moreover, it notably decreased β-catenin protein levels, resulting in an augmented occurrence of cells displaying abnormal spindle segregation during mitosis (P=0.04).
EEROP treatment reduces β-catenin protein levels, promotes abnormal spindle apparatus segregation, and finally leads to apoptotic cell death in CRC cells.
Copyright: © Iranian Journal of Medical Sciences.
Product Citations: 36
In Iranian Journal of Medical Sciences on 1 December 2024 by Dewi, F. R. P., Wahyuningsih, S. P. A., et al.
-
Homo sapiens (Human)
-
Cancer Research
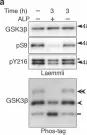
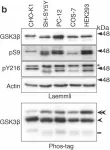
The Axin scaffold protects the kinase GSK3β from cross-pathway inhibition.
In eLife on 7 August 2023 by Gavagan, M., Jameson, N., et al.
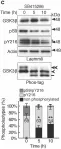
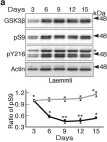
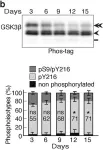
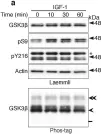
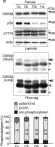
Multiple signaling pathways regulate the kinase GSK3β by inhibitory phosphorylation at Ser9, which then occupies the GSK3β priming pocket and blocks substrate binding. Since this mechanism should affect GSK3β activity toward all primed substrates, it is unclear why Ser9 phosphorylation does not affect other GSK3β-dependent pathways, such as Wnt signaling. We used biochemical reconstitution and cell culture assays to evaluate how Wnt-associated GSK3β is insulated from cross-activation by other signals. We found that the Wnt-specific scaffold protein Axin allosterically protects GSK3β from phosphorylation at Ser9 by upstream kinases, which prevents accumulation of pS9-GSK3β in the Axin•GSK3β complex. Scaffold proteins that protect bound proteins from alternative pathway reactions could provide a general mechanism to insulate signaling pathways from improper crosstalk.
© 2023, Gavagan et al.
In Proceedings of the National Academy of Sciences of the United States of America on 20 June 2023 by Nguyen, T. T. T., Lu, W., et al.
T cell antigen receptor stimulation induces tyrosine phosphorylation of downstream signaling molecules and the phosphatidylinositol, Ras, MAPK, and PI3 kinase pathways, leading to T cell activation. Previously, we reported that the G-protein-coupled human muscarinic receptor could bypass tyrosine kinases to activate the phosphatidylinositol pathway and induce interleukin-2 production in Jurkat leukemic T cells. Here, we demonstrate that stimulating G-protein-coupled muscarinic receptors (M1 and synthetic hM3Dq) can activate primary mouse T cells if PLCβ1 is coexpressed. Resting peripheral hM3Dq+PLCβ1 (hM3Dq/β1) T cells did not respond to clozapine, an hM3Dq agonist, unless they were preactivated by TCR and CD28 stimulation which increased hM3Dq and PLCβ1 expression. This permitted large calcium and phosphorylated ERK responses to clozapine. Clozapine treatment induced high IFN-γ, CD69, and CD25 expression, but surprisingly did not induce substantial IL-2 in hM3Dq/β1 T cells. Importantly, costimulation of both muscarinic receptors plus the TCR even led to reduced IL-2 expression, suggesting a selective inhibitory effect of muscarinic receptor costimulation. Stimulation of muscarinic receptors induced strong nuclear translocation of NFAT and NFκB and activated AP-1. However, stimulation of hM3Dq led to reduced IL-2 mRNA stability which correlated with an effect on the IL-2 3'UTR activity. Interestingly, stimulation of hM3Dq resulted in reduced pAKT and its downstream pathway. This may explain the inhibitory impact on IL-2 production in hM3Dq/β1T cells. Moreover, an inhibitor of PI3K reduced IL-2 production in TCR-stimulated hM3Dq/β1 CD4 T cells, suggesting that activating the pAKT pathway is critical for IL-2 production in T cells.
-
WB
-
Immunology and Microbiology
In Scientific Reports on 31 May 2023 by Leem, Y. H., Kim, D. Y., et al.
Parkinson's disease (PD) is an incurable movement disorder characterized by dopaminergic cell loss, neuroinflammation, and α-synuclein pathology. Herein, we investigated the therapeutic effects of necrosulfonamide (NSA), a specific inhibitor of mixed lineage kinase domain-like protein (MLKL), in a subacute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of PD. MLKL is an executor of necroptosis, a programmed cell death pathway that causes inflammation. Repeated administration of NSA resulted in the recovery of impaired motor performance and dopaminergic degeneration. Furthermore, NSA inhibited the phosphorylation, ubiquitylation, and oligomerization of MLKL, all of which are associated with MLKL cell death-inducing activity in dopaminergic cells in the substantia nigra (SN). NSA also inhibited microglial activation and reactive astrogliosis as well as the MPTP-induced expression of proinflammatory molecules such as tumor necrosis factor-α, interleukin-1β, inducible nitric oxide synthase, and cystatin F. Furthermore, NSA inhibited α-synuclein oligomerization and phosphorylation in the SN of MPTP-treated mice by inhibiting the activity of glycogen synthase kinase 3β and matrix metalloproteinase-3. In conclusion, NSA has anti-necroptotic, anti-inflammatory, and anti-synucleinopathic effects on PD pathology. Therefore, NSA is a potential therapeutic candidate for PD.
© 2023. The Author(s).
-
Mus musculus (House mouse)
-
Neuroscience
In Cancer Cell on 13 March 2023 by Klement, J. D., Redd, P. S., et al.
The cellular and molecular mechanisms underlying tumor cell PD-L1 (tPD-L1) function in tumor immune evasion are incompletely understood. We report here that tPD-L1 does not suppress cytotoxic T lymphocyte (CTL) activity in co-cultures of tumor cells and tumor-specific CTLs and exhibits no effect on primary tumor growth. However, deleting tPD-L1 decreases lung metastasis in a CTL-dependent manner in tumor-bearing mice. Depletion of myeloid cells or knocking out PD-1 in myeloid cells (mPD-1) impairs tPD-L1 promotion of tumor lung metastasis in mice. Single-cell RNA sequencing (scRNA-seq) reveals that tPD-L1 engages mPD-1 to activate SHP2 to antagonize the type I interferon (IFN-I) and STAT1 pathway to repress Cxcl9 and impair CTL recruitment to lung metastases. Human cancer patient response to PD-1 blockade immunotherapy correlates with IFN-I response in myeloid cells. Our findings determine that tPD-L1 engages mPD-1 to activate SHP2 to suppress the IFN-I-STAT1-CXCL9 pathway to impair CTL tumor recruitment in lung metastasis.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
-
WB
-
Mus musculus (House mouse)
-
Cancer Research
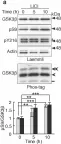
In Elife on 16 January 2020 by Zhou, L., Shao, C. Y., et al.
Fig.8.A

-
WB
-
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 13
In Sci Rep on 17 August 2017 by Krishnankutty, A., Kimura, T., et al.
Fig.2.A

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 13
In Sci Rep on 17 August 2017 by Krishnankutty, A., Kimura, T., et al.
Fig.2.B

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 13
In Sci Rep on 17 August 2017 by Krishnankutty, A., Kimura, T., et al.
Fig.3.A

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 13
In Sci Rep on 17 August 2017 by Krishnankutty, A., Kimura, T., et al.
Fig.3.C

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 13
In Sci Rep on 17 August 2017 by Krishnankutty, A., Kimura, T., et al.
Fig.5.A

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 13
In Sci Rep on 17 August 2017 by Krishnankutty, A., Kimura, T., et al.
Fig.5.B

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 13
In Sci Rep on 17 August 2017 by Krishnankutty, A., Kimura, T., et al.
Fig.6.A

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 13
In Sci Rep on 17 August 2017 by Krishnankutty, A., Kimura, T., et al.
Fig.7.B

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 13
In Sci Rep on 17 August 2017 by Krishnankutty, A., Kimura, T., et al.
Fig.7.C

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 13
In Sci Rep on 17 August 2017 by Krishnankutty, A., Kimura, T., et al.
Fig.8.A

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 13
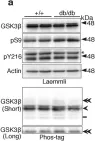
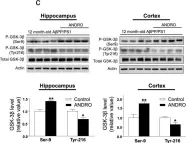
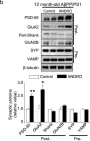
In Mol Neurodegener on 18 December 2014 by Serrano, F. G., Tapia-Rojas, C., et al.
Fig.3.C

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Mol Neurodegener by CiteAb, provided under a CC-BY license
Image 1 of 13
In Mol Neurodegener on 18 December 2014 by Serrano, F. G., Tapia-Rojas, C., et al.
Fig.3.B

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Mol Neurodegener by CiteAb, provided under a CC-BY license
Image 1 of 13