Abstract The molecular basis for accelerated cognitive decline seen in Alzheimer’s Disease (AD) cases presenting with cortical alpha-Synuclein (SNCA/⍺-Syn) co-pathology is not well understood. We show that such AD co-pathology brains are characterized by an increased polygenic risk score for Parkinson’s Disease (PD), which is related to an enrichment in the MAPT H1 haplotype as well as risk factors known to increase SNCA transcription. AD + ASYN brains express higher levels of ⍺-Syn and neuronal microtubule-associated protein tau (MAPT), and increasing SNCA expression is sufficient to drive transcription, translation and phosphorylation of tau. In addition, tau is significantly elevated in subjects with a positive cerebrospinal fluid ⍺-Syn seeding aggregation assay. Our results reveal a hitherto unknown link between the pathogenesis of AD and PD whereby tau and ⍺-Syn synergistically drive dementia-related pathology.
Product Citations: 34
Alpha-Synuclein co-pathology in Alzheimer’s Disease drives tau accumulation
Preprint on Research Square on 21 May 2025 by Struebing, F. L., Song, X., et al.
-
WB
-
Neuroscience
-
Pathology
In Translational Neurodegeneration on 2 August 2024 by Li, L. J., Sun, X. Y., et al.
Deoxyribonuclease 2 (DNase II) plays a key role in clearing cytoplasmic double-stranded DNA (dsDNA). Deficiency of DNase II leads to DNA accumulation in the cytoplasm. Persistent dsDNA in neurons is an early pathological hallmark of senescence and neurodegenerative diseases including Alzheimer's disease (AD). However, it is not clear how DNase II and neuronal cytoplasmic dsDNA influence neuropathogenesis. Tau hyperphosphorylation is a key factor for the pathogenesis of AD. The effect of DNase II and neuronal cytoplasmic dsDNA on neuronal tau hyperphosphorylation remains unclarified.
The levels of neuronal DNase II and dsDNA in WT and Tau-P301S mice of different ages were measured by immunohistochemistry and immunolabeling, and the levels of DNase II in the plasma of AD patients were measured by ELISA. To investigate the impact of DNase II on tauopathy, the levels of phosphorylated tau, phosphokinase, phosphatase, synaptic proteins, gliosis and proinflammatory cytokines in the brains of neuronal DNase II-deficient WT mice, neuronal DNase II-deficient Tau-P301S mice and neuronal DNase II-overexpressing Tau-P301S mice were evaluated by immunolabeling, immunoblotting or ELISA. Cognitive performance was determined using the Morris water maze test, Y-maze test, novel object recognition test and open field test.
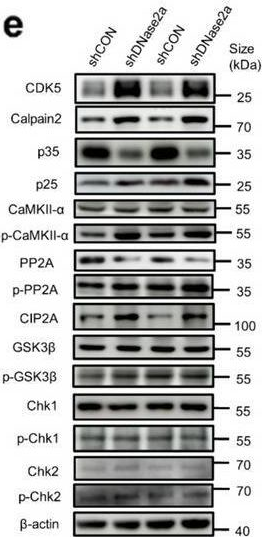
The levels of DNase II were significantly decreased in the brains and the plasma of AD patients. DNase II also decreased age-dependently in the neurons of WT and Tau-P301S mice, along with increased dsDNA accumulation in the cytoplasm. The DNA accumulation induced by neuronal DNase II deficiency drove tau phosphorylation by upregulating cyclin-dependent-like kinase-5 (CDK5) and calcium/calmodulin activated protein kinase II (CaMKII) and downregulating phosphatase protein phosphatase 2A (PP2A). Moreover, DNase II knockdown induced and significantly exacerbated neuron loss, neuroinflammation and cognitive deficits in WT and Tau-P301S mice, respectively, while overexpression of neuronal DNase II exhibited therapeutic benefits.
DNase II deficiency and cytoplasmic dsDNA accumulation can initiate tau phosphorylation, suggesting DNase II as a potential therapeutic target for tau-associated disorders.
© 2024. The Author(s).
-
WB
-
Genetics
-
Neuroscience
Neuronal downregulation of PLCG2 impairs synaptic function and elicits Alzheimer disease hallmarks
Preprint on Research Square on 31 May 2024 by Lambert, J., Coulon, A., et al.
Abstract We developed a high content screening to investigate how Alzheimer disease (AD) genetic risk factors may impair synaptic mechanisms in rat primary neuronal cultures. Out of the gene targets identified, we found that shRNA-mediated downregulation of Plcg2 in mouse dentate gyrus neurons consistently impaired dendritic morphology and synaptic function. In human neuronal cultures (hNCs), PLCG2 downregulation also impaired synaptic function and was associated with increased levels of Aβ and Tau phosphorylation, potentially via the AKT/GSK3β axis. Very rare PLCG2 loss-of-functon(LoF) variants were associated with a 10-fold increased AD risk. PLCG2 LoF carriers exhibit low mRNA/protein PLCG2/PLCγ2 levels, consistent with nonsense-mediated mRNA decay mechanisms. Restoring PLCγ2 levels in shPLCG2-hNCs fully reversed the disease-related phenotypes. Our findings indicate that the downregulation of PLCγ2 increases the risk of AD by impairing synaptic function and increasing the levels of Aβ and Tau phosphorylation in neurons.
-
WB
-
Neuroscience
In Cell Death & Disease on 15 February 2023 by Tseng, K. Y., Stratoulias, V., et al.
During intracerebral hemorrhage (ICH), hematoma formation at the site of blood vessel damage results in local mechanical injury. Subsequently, erythrocytes lyse to release hemoglobin and heme, which act as neurotoxins and induce inflammation and secondary brain injury, resulting in severe neurological deficits. Accelerating hematoma resorption and mitigating hematoma-induced brain edema by modulating immune cells has potential as a novel therapeutic strategy for functional recovery after ICH. Here, we show that intracerebroventricular administration of recombinant human cerebral dopamine neurotrophic factor (rhCDNF) accelerates hemorrhagic lesion resolution, reduces peri-focal edema, and improves neurological outcomes in an animal model of collagenase-induced ICH. We demonstrate that CDNF acts on microglia/macrophages in the hemorrhagic striatum by promoting scavenger receptor expression, enhancing erythrophagocytosis and increasing anti-inflammatory mediators while suppressing the production of pro-inflammatory cytokines. Administration of rhCDNF results in upregulation of the Nrf2-HO-1 pathway, but alleviation of oxidative stress and unfolded protein responses in the perihematomal area. Finally, we demonstrate that intravenous delivery of rhCDNF has beneficial effects in an animal model of ICH and that systemic application promotes scavenging by the brain's myeloid cells for the treatment of ICH.
© 2023. The Author(s).
-
WB
-
Cell Biology
-
Immunology and Microbiology
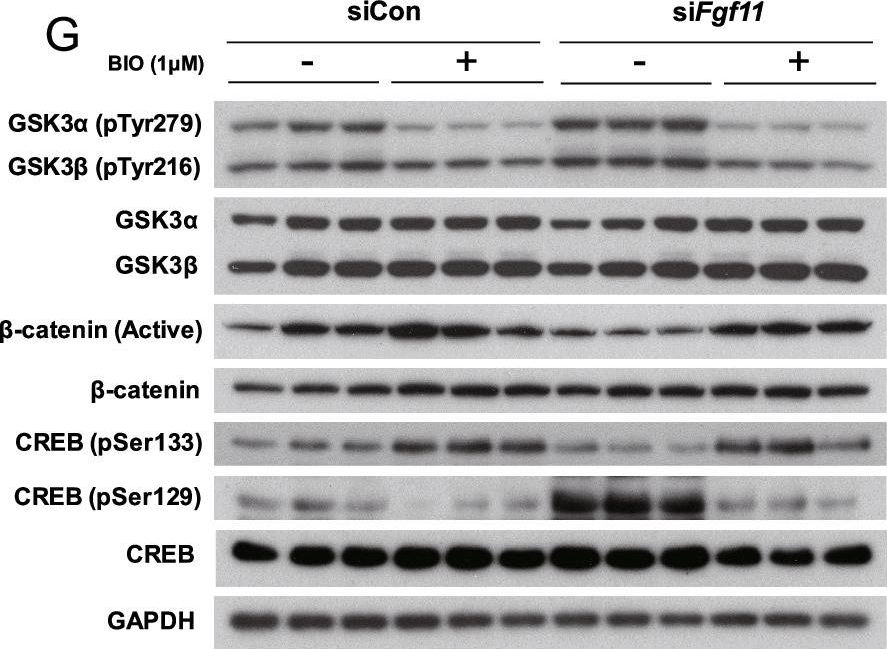
Silencing of hypothalamic FGF11 prevents diet-induced obesity.
In Molecular Brain on 5 September 2022 by Cho, J. H., Kim, K., et al.
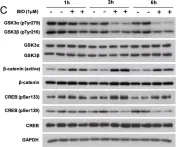
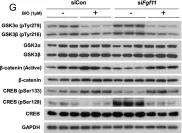
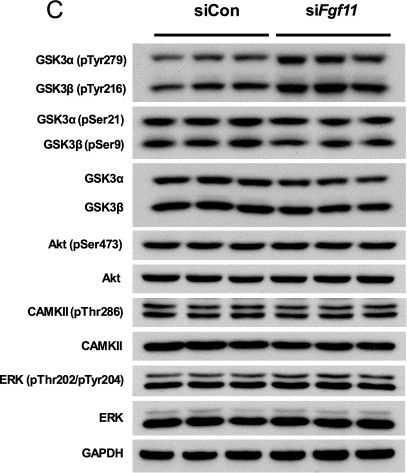
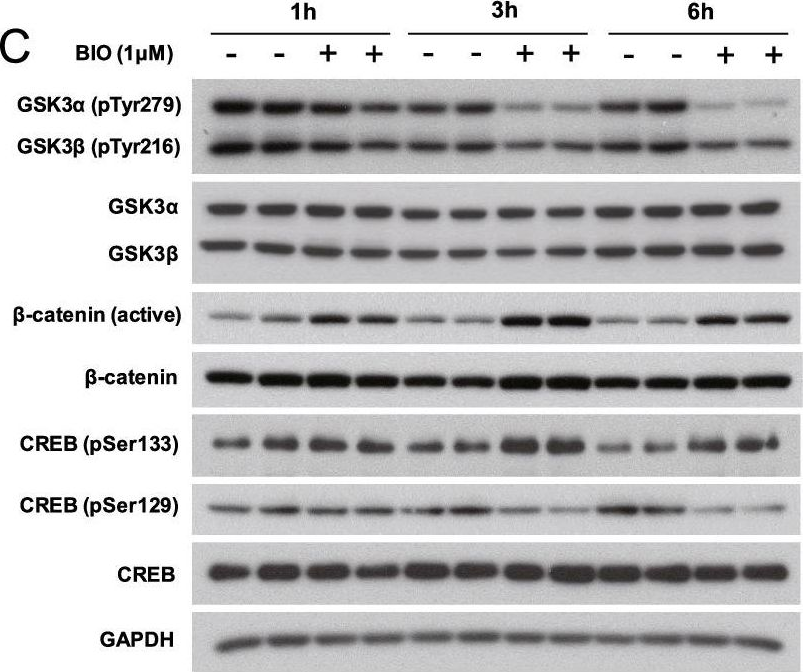
Fibroblast growth factor 11 (FGF11) is a member of the intracellular fibroblast growth factor family. Here, we report the central role of FGF11 in the regulation of metabolism. Lentiviral injection of Fgf11 shRNA into the arcuate nucleus of the mouse hypothalamus decreased weight gain and fat mass, increased brown adipose tissue thermogenesis, and improved glucose and insulin intolerances under high-fat diet conditions. Fgf11 was expressed in the NPY-expressing neurons, and Fgf11 knockdown considerably decreased Npy expression and projection, leading to increased expression of tyrosine hydroxylase in the paraventricular nucleus. Mechanistically, FGF11 regulated Npy gene expression through the glycogen synthase kinase 3-cAMP response element-binding protein pathway. Our study defines the physiological significance of hypothalamic FGF11 in the regulation of metabolism in response to overnutrition such as high-fat diet.
© 2022. The Author(s).
-
WB
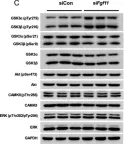
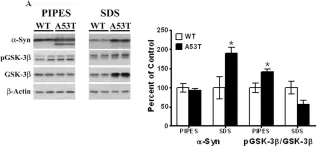
In Transl Neurodegener on 2 August 2024 by Li, L. J., Sun, X. Y., et al.
Fig.2.E
-
WB
-
Collected and cropped from Translational Neurodegeneration by CiteAb, provided under a CC-BY license
Image 1 of 10
In Mol Brain on 5 September 2022 by Cho, J. H., Kim, K., et al.
Fig.5.C
-
WB
-
Collected and cropped from Molecular Brain by CiteAb, provided under a CC-BY license
Image 1 of 10
In Mol Brain on 5 September 2022 by Cho, J. H., Kim, K., et al.
Fig.6.C
-
WB
-
Collected and cropped from Molecular Brain by CiteAb, provided under a CC-BY license
Image 1 of 10
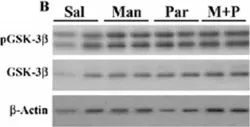
In Mol Brain on 5 September 2022 by Cho, J. H., Kim, K., et al.
Fig.6.G
-
WB
-
Collected and cropped from Molecular Brain by CiteAb, provided under a CC-BY license
Image 1 of 10
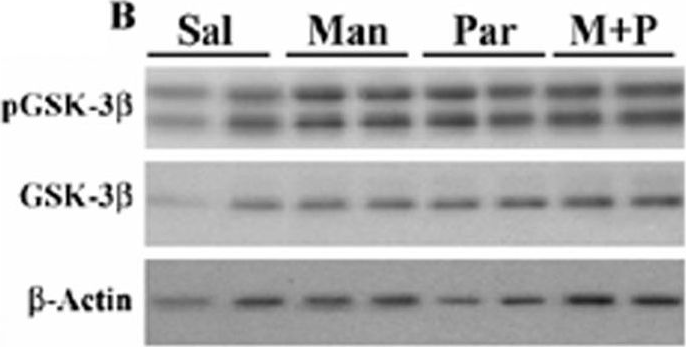
In PLoS One on 1 February 2012 by Wills, J., Credle, J., et al.
Fig.1.B
-
WB
-
Collected and cropped from PLoS ONE by CiteAb, provided under a CC-BY license
Image 1 of 10
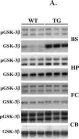
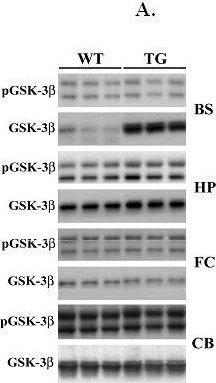
In BMC Neurosci on 3 August 2011 by Kaul, T., Credle, J., et al.
Fig.4.A
-
WB
-
Mus musculus (House mouse)
Collected and cropped from BMC Neuroscience by CiteAb, provided under a CC-BY license
Image 1 of 10
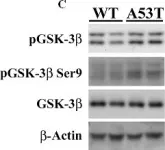
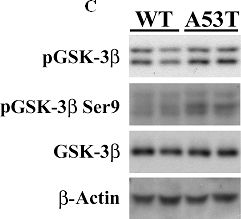
In PLoS One on 30 March 2011 by Wills, J., Credle, J., et al.
Fig.1.C
-
WB
-
Mus musculus (House mouse)
Collected and cropped from PLoS ONE by CiteAb, provided under a CC-BY license
Image 1 of 10
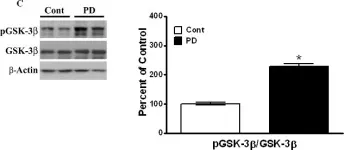
In PLoS One on 30 March 2011 by Wills, J., Credle, J., et al.
Fig.2.C
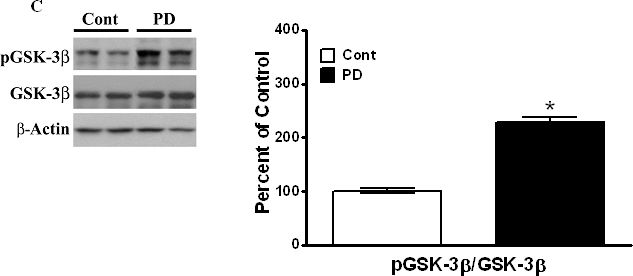
-
WB
-
Mus musculus (House mouse)
Collected and cropped from PLoS ONE by CiteAb, provided under a CC-BY license
Image 1 of 10
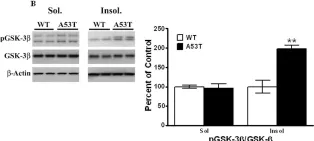
In PLoS One on 30 March 2011 by Wills, J., Credle, J., et al.
Fig.3.B
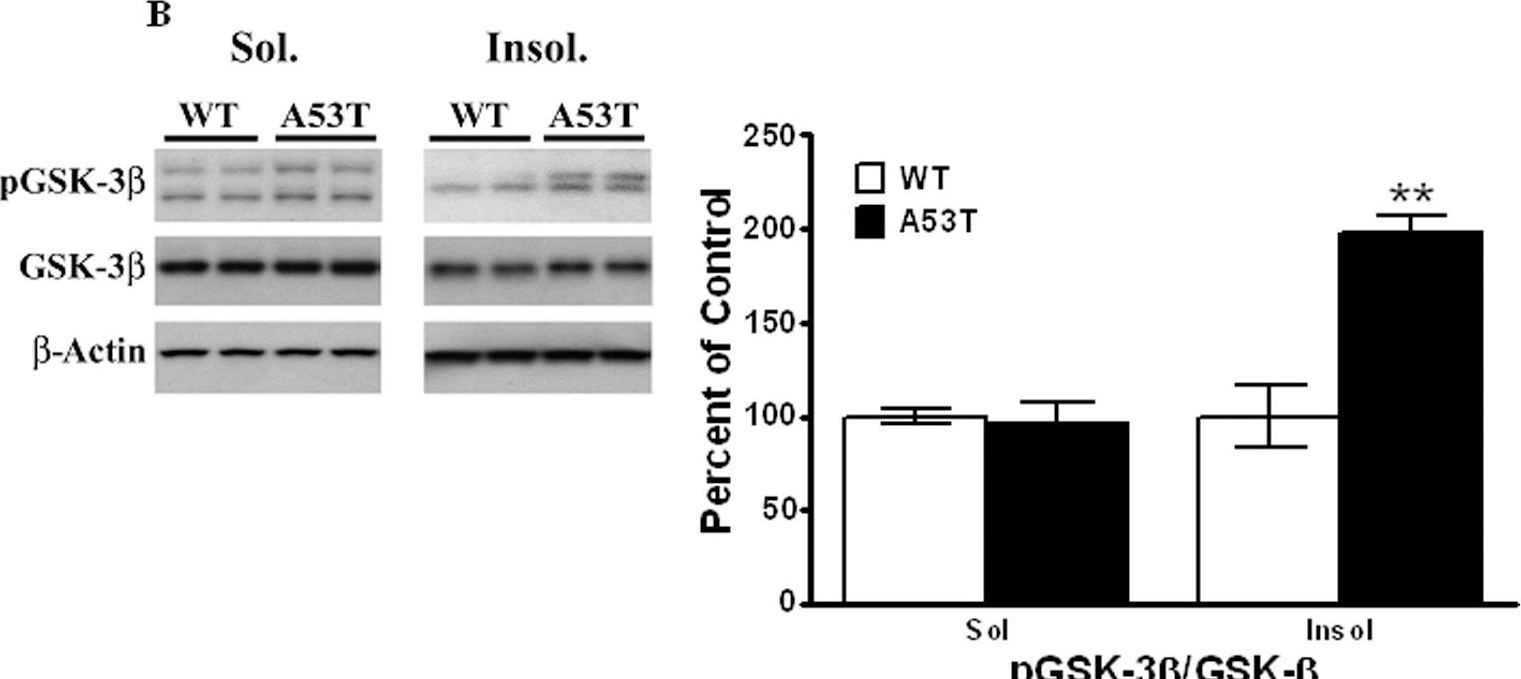
-
WB
-
Collected and cropped from PLoS ONE by CiteAb, provided under a CC-BY license
Image 1 of 10
In PLoS One on 30 March 2011 by Wills, J., Credle, J., et al.
Fig.4.A
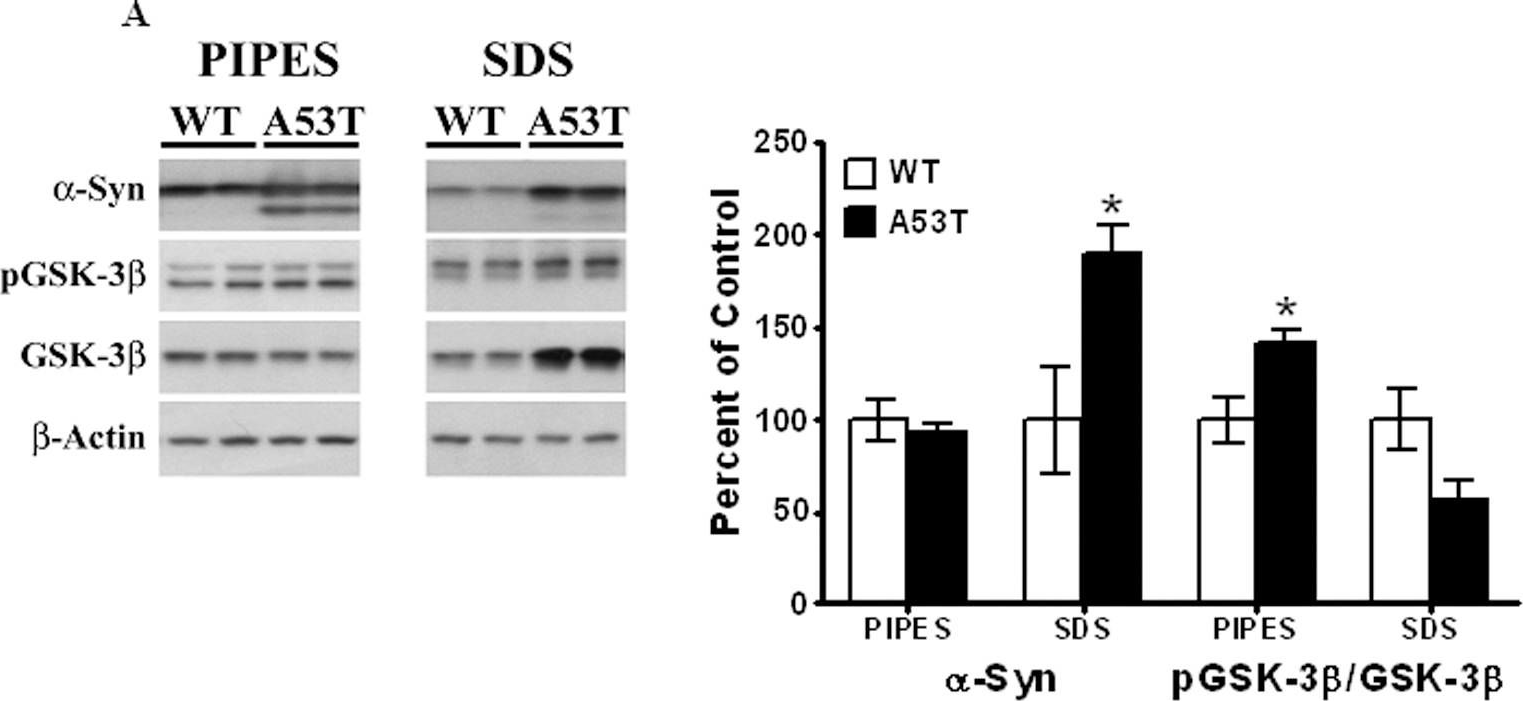
-
WB
-
Collected and cropped from PLoS ONE by CiteAb, provided under a CC-BY license
Image 1 of 10