Necrotizing enterocolitis (NEC) is a devastating disease of premature infants, whose pathogenesis remains incompletely understood, although activation of the Gram-negative bacterial receptor Toll-like receptor 4 (TLR4) on the intestinal epithelium plays a critical role. Patients with NEC typically display gastrointestinal dysmotility before systemic disease is manifest, suggesting that dysmotility could drive NEC development. Both intestinal motility and inflammation are governed by the enteric nervous system, a network of enteric neurons and glia. We hypothesized here that enteric glia loss in the premature intestine could lead to dysmotility, exaggerated TLR4 signaling, and NEC development. We found that intestinal motility is reduced early in NEC in mice, preceding the onset of intestinal inflammation, whereas pharmacologic restoration of intestinal motility reduced NEC severity. Ileal samples from mouse, piglet, and human NEC revealed enteric glia depletion, and glia-deficient mice (Plp1ΔDTR, Sox10ΔDTR, and BdnfΔDTR) showed increased NEC severity compared with wild-type mice. Mice lacking TLR4 on enteric glia (Sox10-Tlr4ko) did not show NEC-induced enteric glia depletion and were protected from NEC. Mechanistically, brain-derived neurotrophic factor (BDNF) from enteric glia restrained TLR4 signaling on the intestine to prevent NEC. BDNF was reduced in mouse and human NEC, and BDNF administration reduced both TLR4 signaling and NEC severity in enteric glia–deficient mice. Last, we identified an agent (J11) that enhanced enteric glial BDNF release, inhibited intestinal TLR4, restored motility, and prevented NEC in mice. Thus, enteric glia loss might contribute to NEC through intestinal dysmotility and increased TLR4 activation, suggesting enteric glia therapies for this disorder.
Product Citations: 8
In Science Translational Medicine on 22 September 2021 by Kovler, M. L., Gonzalez Salazar, A. J., et al.
-
Neuroscience
In PLoS ONE on 20 August 2021 by Alasady, M. J., Terry, A. R., et al.
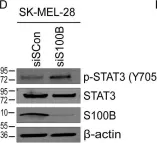
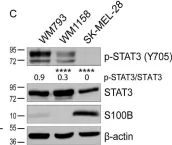
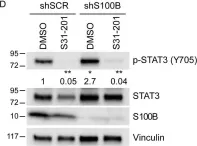
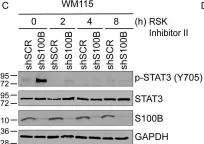
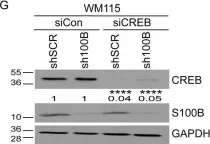
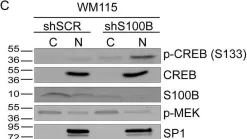
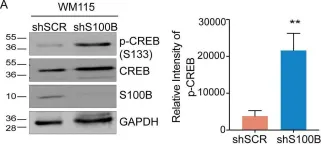
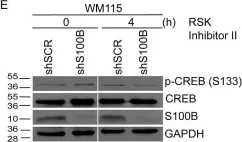
S100B is frequently elevated in malignant melanoma. A regulatory mechanism was uncovered here in which elevated S100B lowers mRNA and secreted protein levels of interleukin-6 (IL6) and inhibits an autocrine loop whereby IL6 activates STAT3 signaling. Our results showed that S100B affects IL6 expression transcriptionally. S100B was shown to form a calcium-dependent protein complex with the p90 ribosomal S6 kinase (RSK), which in turn sequesters RSK into the cytoplasm. Consistently, S100B inhibition was found to restore phosphorylation of a nuclear located RSK substrate, CREB, which is a potent transcription factor for IL6 expression. Thus, elevated S100B reduces IL6-STAT3 signaling via RSK signaling pathway in malignant melanoma. Indeed, the elevated S100B levels in malignant melanoma cell lines correspond to low levels of IL6 and p-STAT3.
-
WB
-
Cancer Research
Unsupervised machine learning reveals risk stratifying glioblastoma tumor cells.
In eLife on 23 June 2020 by Leelatian, N., Sinnaeve, J., et al.
A goal of cancer research is to reveal cell subsets linked to continuous clinical outcomes to generate new therapeutic and biomarker hypotheses. We introduce a machine learning algorithm, Risk Assessment Population IDentification (RAPID), that is unsupervised and automated, identifies phenotypically distinct cell populations, and determines whether these populations stratify patient survival. With a pilot mass cytometry dataset of 2 million cells from 28 glioblastomas, RAPID identified tumor cells whose abundance independently and continuously stratified patient survival. Statistical validation within the workflow included repeated runs of stochastic steps and cell subsampling. Biological validation used an orthogonal platform, immunohistochemistry, and a larger cohort of 73 glioblastoma patients to confirm the findings from the pilot cohort. RAPID was also validated to find known risk stratifying cells and features using published data from blood cancer. Thus, RAPID provides an automated, unsupervised approach for finding statistically and biologically significant cells using cytometry data from patient samples.
© 2020, Leelatian et al.
-
Homo sapiens (Human)
-
Cancer Research
S100B Is a Potential Disease Activity Marker in Nonsegmental Vitiligo.
In The Journal of Investigative Dermatology on 1 July 2017 by Speeckaert, R., Voet, S., et al.
Vitiligo is a chronic skin condition characterized by progressive depigmentation of the skin. S100B is a damage-associated molecular pattern protein expressed in melanocytes that has been proposed as a marker of melanocyte cytotoxicity. Although the use of S100B as a biomarker in melanoma is well established, to our knowledge its association with vitiligo activity has not yet been investigated. Here, we show that S100B serum levels were significantly increased in patients with active nonsegmental vitiligo and strongly correlated with the affected body surface area. Prospective follow-up showed a predictive value of serum S100B levels on disease progression. In vitro experiments using repeated freeze-thaw procedures showed an intracellular up-regulation of S100B in normal and vitiligo melanocytes before an extensive release in the environment. This phenomenon may explain the increased S100B serum values in the active phase of vitiligo. In a monobenzone-induced vitiligo mouse model we could show the potential of S100B inhibition as a therapeutic strategy in vitiligo. In conclusion, this report shows the possible use of S100B as a biomarker for disease activity in vitiligo. Our data suggest that this damage-associated molecular pattern protein could play a substantial role in the pathogenesis of vitiligo and may be a potential new target for treatment.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
-
FC/FACS
In European Journal of Immunology on 1 February 2016 by van Nierop, G. P., Janssen, M., et al.
MS pathology is potentially orchestrated by autoreactive T cells, but the antigens recognized remain unknown. A novel APC/T-cell platform was developed to determine intrathecal CD4(+) and CD8(+) T-cell responses to candidate MS-associated autoantigens (cMSAg) in clinically isolated syndrome (CIS, n = 7) and MS (n = 6) patients. Human cMSAg encoding open reading frames (n = 8) were cloned into an Epstein-Barr virus (EBV)-based vector to express cMSAg at high levels in EBV-transformed B-cells (BLCLs). Human cMSAg cloned were myelin-associated and -oligodendrocyte glycoprotein, myelin basic protein, proteolipid protein, ATP-dependent potassium channel ATP-dependent inwards rectifying potassium channel 4.1, S100 calcium-binding protein B, contactin-2, and neurofascin. Transduced BLCLs were used as autologous APC in functional T-cell assays to determine cMSAg-specific T-cell frequencies in cerebrospinal fluid derived T-cell lines (CSF-TCLs) by intracellular IFN-γ flow cytometry. Whereas all CSF-TCL responded strongly to mitogenic stimulation, no substantial T-cell reactivity to cMSAg was observed. Contrastingly, measles virus fusion protein-specific CD4(+) and CD8(+) T-cell clones, used as control of the APC/T-cell platform, efficiently recognized transduced BLCL expressing their cognate antigen. The inability to detect substantial T-cell reactivity to eight human endogenously synthesized cMSAg in autologous APC do not support their role as prominent intrathecal T-cell target antigens in CIS and MS patients early after onset of disease.
© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
-
Immunology and Microbiology
In PLoS One on 20 August 2021 by Alasady, M. J., Terry, A. R., et al.
Fig.2.D

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 8
In PLoS One on 20 August 2021 by Alasady, M. J., Terry, A. R., et al.
Fig.2.C

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 8
In PLoS One on 20 August 2021 by Alasady, M. J., Terry, A. R., et al.
Fig.3.D

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 8
In PLoS One on 20 August 2021 by Alasady, M. J., Terry, A. R., et al.
Fig.5.C

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 8
In PLoS One on 20 August 2021 by Alasady, M. J., Terry, A. R., et al.
Fig.6.G

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 8
In PLoS One on 20 August 2021 by Alasady, M. J., Terry, A. R., et al.
Fig.6.C

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 8
In PLoS One on 20 August 2021 by Alasady, M. J., Terry, A. R., et al.
Fig.6.A

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 8
In PLoS One on 20 August 2021 by Alasady, M. J., Terry, A. R., et al.
Fig.6.E

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 8