Eukaryotic elongation factor 2 kinase (eEF2K) belongs to the Ca2+/calmodulin-dependent protein kinase family. We previously revealed that A484954, a selective eEF2K inhibitor, caused hypotensive and diuretic effects via the production of nitric oxide (NO) in spontaneously hypertensive rats. Otsuka Long-Evans Tokushima Fatty (OLETF) rats are hypertensive because of obesity and type 2 diabetes. Because an NO synthase inhibitor was reported to increase the expression of sodium glucose co-transporter 2 (SGLT2), we hypothesised that A484954 causes not only hypotensive but also hypoglycaemic effects via NO production in OLETF rats.
To test the hypothesis, we examined the effects of A484954 administration on hyperglycaemia and hypertension in OLETF rats. OLETF rats were given an intraperitoneal injection of A484954 (2.5 mg kg-1 day-1) for 7 days. Then, we measured blood and urinary glucose level, urine output, systolic blood pressure and ventricular contractility. We also conducted Western blotting and isometric tension measurements.
A484954 induced a decrease in blood glucose, an increase in urinary glucose excretion, and a decrease in protein expression of kidney SGLT2. In addition, A484954 induced a decrease in systolic blood pressure, an NO-dependent vasorelaxation, and a diuretic effect. Further, A484954 enhanced left ventricular contractility.
We, for the first time, revealed that (1) A484954 caused hypoglycaemic effects through increasing urinary glucose excretion via decreasing SGLT2, (2) A484954 improved diabetic complication, including hypertension, through vasorelaxation and diuresis via NO production, and (3) A484954 had a positive inotropic effect.
© 2025 British Pharmacological Society.
Product Citations: 55
In British Journal of Pharmacology on 1 April 2025 by Kodama, T., Kameshima, S., et al.
-
Pharmacology
In Scientific Reports on 13 February 2024 by Böttner, J., Fischer-Schaepmann, T., et al.
Abuse of amphetamine-type stimulants is linked to cardiovascular adverse effects like arrhythmias, accelerated atherosclerosis, acute coronary syndromes and sudden cardiac death. Excessive catecholamine release following amphetamine use causes vasoconstriction and vasospasms, over time leading to hypertension, endothelial dysfunction or even cardiotoxicity. However, immediate vascular pathomechanisms related to amphetamine exposure, especially endothelial function, remain incompletely understood and were analyzed in this study. Pharmaco-pathological effects of acute d-amphetamine-sulfate (DAM) were investigated ex vivo using contraction-force measurements of rat carotid artery rings and in vitro using label-free, real-time electrochemical impedance spectroscopy (EIS) on endothelial and smooth muscle cells. Specific receptor and target blocking was used to identify molecular targets and to characterize intracellular signaling. DAM induced vasodilation represented by 29.3±2.5% decrease in vascular tone (p<0.001) involving vascular endothelial growth factor receptor (VEGF-R) and protease activated receptor 1 (PAR-1). EIS revealed that DAM induces endothelial barrier disruption (-75.9±1.1% of initial cellular impedance, p<0.001) also involving VEGF-R and PAR-1. Further, in response to DAM, Rho-associated protein kinase (ROCK) mediated reversible contraction of actin cytoskeleton resulting in endothelial barrier disruption. Dephosphorylation of Serine1177 (-50.8±3.7%, p<0.001) and Threonine495 (-44.8±6.5%, p=0.0103) of the endothelial NO synthase (eNOS) were also observed. Blocking of VEGF-R and PAR-1 restored baseline eNOS Threonine495 phosphorylation. DAM induced vasodilation, enhanced vascular permeability and actin cytoskeleton contraction and induced eNOS hypophosphorylation involving VEGF-R, PAR-1 and ROCK. These results may contribute to a better understanding of severe adverse cardiovascular effects in amphetamine abuse.
© 2024. The Author(s).
-
Cell Biology
In The Journal of Veterinary Medical Science / the Japanese Society of Veterinary Science on 19 December 2023 by Kodama, T., Otani, K., et al.
Eukaryotic elongation factor 2 (eEF2) kinase (eEF2K) is a protein kinase that inactivates eEF2, a protein that mediates a peptidyl-tRNA translocation during an elongation step of protein synthesis. We have previously shown that eEF2K was involved in pathogenesis of essential and pulmonary hypertension. A484954 (7-amino-1-cyclopropyl-3-ethyl-2,4-dioxo-1,2,3,4-tetrahydropyrido[2,3-d] pyrimidine-6-carboxamide), a selective eEF2K inhibitor, is a membrane permeable small molecule. We have previously shown that A484954 lowered blood pressure and induced diuretic effects in spontaneously hypertensive rats (SHR) due to an increase in renal blood flow. Here we aimed to reveal mechanisms underlying the diuretic effects of A484954 in SHR. A484954-induced diuresis and increase in urinary Na+ excretion were inhibited by N (G)-nitro-L-arginine methyl ester (L-NAME), a nitric oxide (NO) synthase inhibitor. A484954 increased mRNA expression of angiotensin type 2 receptor (AT2R) and nuclear factor-erythroid 2-related factor 2 (Nrf2). In summary, we for the first time revealed that A484954 induces diuresis in SHR at least partly via the activation of NO/Nrf2/AT2R pathway.
-
Cardiovascular biology
-
Veterinary Research
In American Journal of Physiology - Cell Physiology on 1 November 2023 by Yu, B., Shen, K., et al.
Glomerular angiogenesis is a characteristic feature of diabetic nephropathy (DN). Enhanced glycolysis plays a crucial role in angiogenesis. The present study was designed to investigate the role of glycolysis in glomerular endothelial cells (GECs) in a mouse model of DN. Mouse renal cortex and isolated glomerular cells were collected for single-cell and RNA sequencing. Cultured GECs were exposed to high glucose in the presence (proangiogenic) and absence of a vascular sprouting regimen. MicroRNA-590-3p was delivered by lipofectamine in vivo and in vitro. In the present study, a subgroup of GECs with proangiogenic features was identified in diabetic kidneys by using sequencing analyses. In cultured proangiogenic GECs, high glucose increased glycolysis and phosphofructokinase/fructose bisphosphatase 3 (PFKFB3) protein expression, which were inhibited by overexpressing miRNA-590-3p. Mimics of miRNA-590-3p also increased receptor for sphingosine 1-phosphate (S1pR1) expression, an angiogenesis regulator, in proangiogenic GECs challenged with high glucose. Inhibition of PFKFB3 by pharmacological and genetic approaches upregulated S1pR1 protein in vitro. Mimics of miRNA-590-3p significantly reduced migration and angiogenic potential in proangiogenic GECs challenged with high glucose. Ten-week-old type 2 diabetic mice had elevated urinary albumin levels, reduced renal cortex miRNA-590-3p expression, and disarrangement of glomerular endothelial cell fenestration. Overexpressing miRNA-590-3p via perirenal adipose tissue injection restored endothelial cell fenestration and reduced urinary albumin levels in diabetic mice. Therefore, the present study identifies a subgroup of GECs with proangiogenic features in mice with DN. Local administration of miRNA-590-3p mimics reduces glycolytic rate and upregulates S1pR1 protein expression in proangiogenic GECs. The protective effects of miRNA-590-3p provide therapeutic potential in DN treatment.NEW & NOTEWORTHY Proangiogenetic glomerular endothelial cells (GECs) are activated in diabetic nephropathy. High glucose upregulates glycolytic enzyme phosphofructokinase/fructose bisphosphatase 3 (PFKFB3) in proangiogenetic cells. PFKFB3 protects the glomerular filtration barrier by targeting endothelial S1pR1. MiRNA-590-3p restores endothelial cell function and mitigates diabetic nephropathy.
-
WB
-
Endocrinology and Physiology
In The Journal of Clinical Investigation on 16 October 2023 by Sjöberg, E., Melssen, M., et al.
Endothelial phospholipase Cγ (PLCγ) is essential for vascular development; however, its role in healthy, mature, or pathological vessels is unexplored. Here, we show that PLCγ was prominently expressed in vessels of several human cancer forms, notably in renal cell carcinoma (RCC). High PLCγ expression in clear cell RCC correlated with angiogenic activity and poor prognosis, while low expression correlated with immune cell activation. PLCγ was induced downstream of vascular endothelial growth factor receptor 2 (VEGFR2) phosphosite Y1173 (pY1173). Heterozygous Vegfr2Y1173F/+ mice or mice lacking endothelial PLCγ (Plcg1iECKO) exhibited a stabilized endothelial barrier and diminished vascular leakage. Barrier stabilization was accompanied by decreased expression of immunosuppressive cytokines, reduced infiltration of B cells, helper T cells and regulatory T cells, and improved response to chemo- and immunotherapy. Mechanistically, pY1173/PLCγ signaling induced Ca2+/protein kinase C-dependent activation of endothelial nitric oxide synthase (eNOS), required for tyrosine nitration and activation of Src. Src-induced phosphorylation of VE-cadherin at Y685 was accompanied by disintegration of endothelial junctions. This pY1173/PLCγ/eNOS/Src pathway was detected in both healthy and tumor vessels in Vegfr2Y1173F/+ mice, which displayed decreased activation of PLCγ and eNOS and suppressed vascular leakage. Thus, we believe that we have identified a clinically relevant endothelial PLCγ pathway downstream of VEGFR2 pY1173, which destabilizes the endothelial barrier and results in loss of antitumor immunity.
-
WB
-
Cancer Research
-
Immunology and Microbiology
In Int J Mol Sci on 27 November 2022 by Tseng, S. Y., Chang, H. Y., et al.
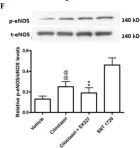
Fig.4.F

-
WB
-
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 2
In Int J Mol Sci on 27 November 2022 by Tseng, S. Y., Chang, H. Y., et al.
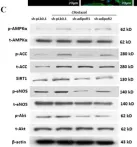
Fig.6.C

-
WB
-
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 2