CD19 and CXCR4 are pivotal regulators of B-cell activation and migration, respectively. Specifically, CXCR4 signaling critically influences the dissemination of various malignant B cells through constitutive activation and aberrant expression. This study explores the interaction between CD19 and CXCR4 signaling in the context of B-cell lymphomas, particularly focusing on diffuse large B-cell lymphoma (DLBCL) and Waldenström Macroglobulinemia (WM). We assessed the roles of CD19 in survival and CXCL12-induced migration by using knockout (KO) cells of DLBCL and WM origin alongside evaluating the impact of CD19 monoclonal antibodies (mAbs) on antibody-dependent cell-mediated cytotoxicity (ADCC). Our results highlight that CD19 is important for survival and CXCL12-induced migration, and mAbs variably increase CXCL12-induced migration and enhance ADCC. Additionally, we demonstrate that the endogenous peptide inhibitor of the CXCR4 (EPI-X4) derivative JM#21 effectively inhibits CD19-mediated migration enhancement and promotes ADCC, thereby augmenting the therapeutic efficacy of CD19 mAb-based immunotherapy in lymphoma models. Our study underscores the potential of targeting both CD19 and CXCR4 to refine therapeutic strategies for treating B-cell malignancies, suggesting a synergistic approach could improve clinical outcomes in WM treatment.
Product Citations: 20
In International Journal of Molecular Sciences on 26 February 2025 by Khunti, N., Kumar, M., et al.
-
Cancer Research
-
Immunology and Microbiology
Slow cycling and durable Flt3+ progenitors contribute to hematopoiesis under native conditions.
In The Journal of Experimental Medicine on 1 January 2024 by Solomon, M., Song, B., et al.
The dynamics of the hematopoietic flux responsible for blood cell production in native conditions remains a matter of debate. Using CITE-seq analyses, we uncovered a distinct progenitor population that displays a cell cycle gene signature similar to the one found in quiescent hematopoietic stem cells. We further determined that the CD62L marker can be used to phenotypically enrich this population in the Flt3+ multipotent progenitor (MPP4) compartment. Functional in vitro and in vivo analyses validated the heterogeneity of the MPP4 compartment and established the quiescent/slow-cycling properties of the CD62L- MPP4 cells. Furthermore, studies under native conditions revealed a novel hierarchical organization of the MPP compartments in which quiescent/slow-cycling MPP4 cells sustain a prolonged hematopoietic activity at steady-state while giving rise to other lineage-biased MPP populations. Altogether, our data characterize a durable and productive quiescent/slow-cycling hematopoietic intermediary within the MPP4 compartment and highlight early paths of progenitor differentiation during unperturbed hematopoiesis.
© 2023 Solomon et al.
Structure of the thrombopoietin-MPL receptor complex is a blueprint for biasing hematopoiesis.
In Cell on 14 September 2023 by Tsutsumi, N., Masoumi, Z., et al.
Thrombopoietin (THPO or TPO) is an essential cytokine for hematopoietic stem cell (HSC) maintenance and megakaryocyte differentiation. Here, we report the 3.4 Å resolution cryoelectron microscopy structure of the extracellular TPO-TPO receptor (TpoR or MPL) signaling complex, revealing the basis for homodimeric MPL activation and providing a structural rationalization for genetic loss-of-function thrombocytopenia mutations. The structure guided the engineering of TPO variants (TPOmod) with a spectrum of signaling activities, from neutral antagonists to partial- and super-agonists. Partial agonist TPOmod decoupled JAK/STAT from ERK/AKT/CREB activation, driving a bias for megakaryopoiesis and platelet production without causing significant HSC expansion in mice and showing superior maintenance of human HSCs in vitro. These data demonstrate the functional uncoupling of the two primary roles of TPO, highlighting the potential utility of TPOmod in hematology research and clinical HSC transplantation.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
In IScience on 21 July 2023 by Al-Rashed, F., Haddad, D., et al.
Foamy and inflammatory macrophages play pathogenic roles in metabolic disorders. However, the mechanisms that promote foamy and inflammatory macrophage phenotypes under acute-high-fat feeding (AHFF) remain elusive. Herein, we investigated the role of acyl-CoA synthetase-1 (ACSL1) in favoring the foamy/inflammatory phenotype of monocytes/macrophages upon short-term exposure to palmitate or AHFF. Palmitate exposure induced a foamy/inflammatory phenotype in macrophages which was associated with increased ACSL1 expression. Inhibition/knockdown of ACSL1 in macrophages suppressed the foamy/inflammatory phenotype through the inhibition of the CD36-FABP4-p38-PPARδ signaling axis. ACSL1 inhibition/knockdown suppressed macrophage foaming/inflammation after palmitate stimulation by downregulating the FABP4 expression. Similar results were obtained using primary human monocytes. As expected, oral administration of ACSL1 inhibitor triacsin-C in mice before AHFF normalized the inflammatory/foamy phenotype of the circulatory monocytes by suppressing FABP4 expression. Our results reveal that targeting ACSL1 leads to the attenuation of the CD36-FABP4-p38-PPARδ signaling axis, providing a therapeutic strategy to prevent the AHFF-induced macrophage foaming and inflammation.
© 2023 The Author(s).
-
Immunology and Microbiology
A modifiable universal cotinine-chimeric antigen system of NK cells with multiple targets.
In Frontiers in Immunology on 31 January 2023 by Kang, H. Y., Lee, S. Y., et al.
Natural killer (NK) cells are immune effector cells with outstanding features for adoptive immunotherapy. Immune effector cells with chimeric antigen receptors (CARs) are promising targeted therapeutic agents for various diseases. Because tumor cells exhibit heterogeneous antigen expression and lose cell surface antigen expression during malignant progression, many CARs fixed against only one antigen have limited efficacy and are associated with tumor relapse. To expand the utility of CAR-NK cells, we designed a split and universal cotinine-CAR (Cot-CAR) system, comprising a Cot-conjugator and NK92 cells (α-Cot-NK92 cells) engineered with a CAR containing an anti-Cot-specific single-chain variable fragment and intracellular signaling domain. The efficacy of the Cot-CAR system was assessed in vitro using a cytolysis assay against various tumor cells, and its single- or multiple- utility potential was demonstrated using an in vivo lung metastasis model by injecting A549-Red-Fluc cells. The α-Cot-NK92 cells could switch targets, logically respond to multiple antigens, and tune cytolytic activation through the alteration of conjugators without re-engineering. Therefore the universal Cot-CAR system is useful for enhancing specificity and diversity of antigens, combating relapse, and controlling cytolytic activity. In conclusion, this universal Cot-CAR system reveals that multiple availability and controllability can be generated with a single, integrated system.
Copyright © 2023 Kang, Lee, Kim, Lee, Lee, Cho, Oh, Kim, Park, Han, Kim, Kim, Yoon, Doh, Chung, Hong, Choi and Kim.
-
FC/FACS
-
Immunology and Microbiology
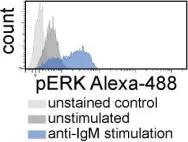
In J Exp Med on 5 February 2018 by Tissino, E., Benedetti, D., et al.
Fig.1.C

-
FC/FACS
-
Homo sapiens (Human)
Collected and cropped from J Exp Med by CiteAb, provided under a CC-BY license
Image 1 of 1