In hepatocytes, the Wilson disease protein ATP7B resides on the trans-Golgi network (TGN) and traffics to peripheral lysosomes to export excess intracellular copper through lysosomal exocytosis. We found that in basal copper or even upon copper chelation, a significant amount of ATP7B persists in the endolysosomal compartment of hepatocytes but not in non-hepatic cells. These ATP7B-harbouring lysosomes lie in close proximity of ~10 nm to the TGN. ATP7B constitutively distributes itself between the sub-domain of the TGN with a lower pH and the TGN-proximal lysosomal compartments. The presence of ATP7B on TGN-lysosome colocalising sites upon Golgi disruption suggested a possible exchange of ATP7B directly between the TGN and its proximal lysosomes. Manipulating lysosomal positioning significantly alters the localisation of ATP7B in the cell. Contrary to previous understanding, we found that upon copper chelation in a copper-replete hepatocyte, ATP7B is not retrieved back to TGN from peripheral lysosomes; rather, ATP7B recycles to these TGN-proximal lysosomes to initiate the next cycle of copper transport. We report a hitherto unknown copper-independent lysosomal localisation of ATP7B and the importance of TGN-proximal lysosomes but not TGN as the terminal acceptor organelle of ATP7B in its retrograde pathway.
© 2023 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Product Citations: 17
Copper-independent lysosomal localisation of the Wilson disease protein ATP7B.
In Traffic (Copenhagen, Denmark) on 1 December 2023 by Maji, S., Pirozzi, M., et al.
-
Cell Biology
In Traffic (Copenhagen, Denmark) on 1 February 2023 by Sumya, F. T., Pokrovskaya, I. D., et al.
Conserved Oligomeric Golgi (COG) complex controls Golgi trafficking and glycosylation, but the precise COG mechanism is unknown. The auxin-inducible acute degradation system was employed to investigate initial defects resulting from COG dysfunction. We found that acute COG inactivation caused a massive accumulation of COG-dependent (CCD) vesicles that carry the bulk of Golgi enzymes and resident proteins. v-SNAREs (GS15, GS28) and v-tethers (giantin, golgin84, and TMF1) were relocalized into CCD vesicles, while t-SNAREs (STX5, YKT6), t-tethers (GM130, p115), and most of Rab proteins remained Golgi-associated. Airyscan microscopy and velocity gradient analysis revealed that different Golgi residents are segregated into different populations of CCD vesicles. Acute COG depletion significantly affected three Golgi-based vesicular coats-COPI, AP1, and GGA, suggesting that COG uniquely orchestrates tethering of multiple types of intra-Golgi CCD vesicles produced by different coat machineries. This study provided the first detailed view of primary cellular defects associated with COG dysfunction in human cells.© 2022 The Authors. Traffic published by John Wiley & Sons Ltd.
-
Cell Biology
Copper-independent lysosomal localization of the Wilson disease protein ATP7B
Preprint on BioRxiv : the Preprint Server for Biology on 1 November 2022 by Maji, S., Ruturaj, et al.
In hepatocytes, the Wilson disease protein, ATP7B resides on trans-Golgi network and traffics to peripheral lysosomes to export excess intracellular copper by means of lysosomal exocytosis. We found that in basal copper or even upon copper chelation, a significant amount of ATP7B persists on endolysosomal compartment of hepatocytes but not in non-hepatic cells. These ATP7B-harboring lysosomes lie in close proximity of < 40 nm to the TGN. ATP7B constitutively distributes itself between the the sub-domain of the TGN with a lower pH and the TGN-proximal lysosomal compartments. Localization of ATP7B on TGN-Lysosome hybrid compartments upon Golgi disruption suggested possible exchange of ATP7B directly between the TGN and its proximal lysosomes. Manipulating lysosomal positioning significantly alters the localization of ATP7B in the cell. Contrary to previous understanding, we found that upon copper chelation in a copper replete hepatocyte, ATP7B is not retrieved back to TGN from peripheral lysosomes; rather ATP7B recycles to these TGN-proximal lysosomes to initiate the next cycle of copper transport. We report a hitherto unknown copper-independent localization of ATP7B i.e., at the lysosomes and also the importance of TGN-proximal lysosomes but not TGN as the terminal acceptor organelle of ATP7B in its retrograde pathway. Synopsis Excess copper is toxic to the cell. In Hepatocytes, the Wilson disease protein, ATP7B has been reported to recycle between the trans-Golgi network and lysosomes to export copper in elevated copper condition. We for the first time show that a large fraction of ATP7B constitutively reside on the lysosome and rather the position of the lysosome shifts from TGN-proximal to peripheral region of the cell in high copper to facilitate ATP7B-mediated lysosomal exocytosis of copper.
-
WB
-
Homo sapiens (Human)
-
Cell Biology
Identification of a Golgi-localized peptide reveals a minimal Golgi-targeting motif.
In Molecular Biology of the Cell on 1 October 2022 by Navarro, A. P. & Cheeseman, I. M.
Prior work has identified signal sequences and motifs that are necessary and sufficient to target proteins to specific subcellular regions and organelles such as the plasma membrane, nucleus, endoplasmic reticulum, and mitochondria. In contrast, minimal sequence motifs that are sufficient for Golgi localization remain largely elusive. In this work, we identified a 37-amino acid alternative open reading frame (altORF) within the mRNA of the centromere protein CENP-R. This altORF peptide localizes specifically to the cytoplasmic surface of the Golgi apparatus. Through mutational analysis, we identify a minimal 10-amino acid sequence and a critical cysteine residue that are necessary and sufficient for Golgi localization. Pharmacological perturbations suggest that this peptide undergoes lipid modification to promote its localization. Together, our work defines a minimal sequence that is sufficient for Golgi targeting and provide a valuable Golgi marker for live cell imaging.
-
Cell Biology
Visualizing intra-Golgi localization and transport by side-averaging Golgi ministacks.
In The Journal of Cell Biology on 6 June 2022 by Tie, H. C., Mahajan, D., et al.
The mammalian Golgi comprises tightly adjacent and flattened membrane sacs called cisternae. We still do not understand the molecular organization of the Golgi and intra-Golgi transport of cargos. One of the most significant challenges to studying the Golgi is resolving Golgi proteins at the cisternal level under light microscopy. We have developed a side-averaging approach to visualize the cisternal organization and intra-Golgi transport in nocodazole-induced Golgi ministacks. Side-view images of ministacks acquired from Airyscan microscopy are transformed and aligned before intensity normalization and averaging. From side-average images of >30 Golgi proteins, we uncovered the organization of the pre-Golgi, cis, medial, trans, and trans-Golgi network membrane with an unprecedented spatial resolution. We observed the progressive transition of a synchronized cargo wave from the cis to the trans-side of the Golgi. Our data support our previous finding, in which constitutive cargos exit at the trans-Golgi while the secretory targeting to the trans-Golgi network is signal dependent.
© 2022 Tie et al.
-
ICC-IF
-
Homo sapiens (Human)
-
Cell Biology
In Life Sci Alliance on 1 March 2022 by Buser, D. P., Bader, G., et al.
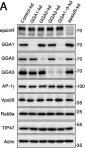
Fig.5.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Life Sci Alliance by CiteAb, provided under a CC-BY license
Image 1 of 4
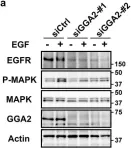
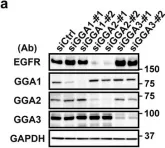
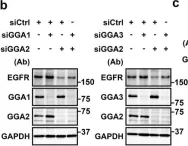
In Sci Rep on 22 January 2018 by Uemura, T., Kametaka, S., et al.
Fig.6.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 4
In Sci Rep on 22 January 2018 by Uemura, T., Kametaka, S., et al.
Fig.1.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 4
In Sci Rep on 22 January 2018 by Uemura, T., Kametaka, S., et al.
Fig.5.B

-
WB
-
Homo sapiens (Human)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 4