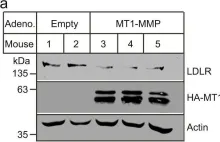
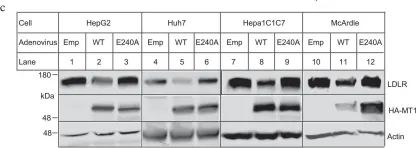
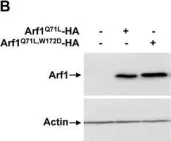
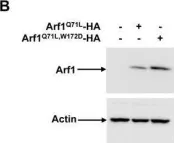
Use-dependent plasticity after spinal cord injury (SCI) enhances neuromotor function, however, the optimal timing to initiate rehabilitation remains controversial. To test impacts of early disuse, we established a rodent model of transient hindlimb suspension in acute phase SCI. Early disuse in the first 2-week after SCI undermined recovery on open-field locomotion, kinematics, and swim tests even after 6-week of normal gravity reloading. Early disuse produced chronic spinal circuit hyper-excitability in H-reflex and interlimb reflex tests. Quantitative synaptoneurosome analysis of lumboventral spinal cords revealed shifts in AMPA receptor (AMPAR) subunit GluA1 localization and serine 881 phosphorylation, reflecting enduring synaptic memories of early disuse stored in the spinal cord. Automated confocal analysis of motoneurons revealed persistent shifts toward GluA2-lacking, calcium-permeable AMPARs in disuse subjects. Unsupervised machine learning associated multidimensional synaptic changes with persistent recovery deficits in SCI. The results argue for early aggressive rehabilitation to prevent disuse plasticity that limits SCI recovery.
© 2025 The Authors.
Product Citations: 85
Disuse plasticity limits spinal cord injury recovery.
In IScience on 18 April 2025 by Morioka, K., Tazoe, T., et al.
-
Neuroscience
Preprint on BioRxiv : the Preprint Server for Biology on 14 April 2025 by Ranganathan, S., Ojo, T., et al.
An unmet challenge in managing breast cancer is treatment failure due to resistance to apoptosis-inducing chemotherapies. Thus, it is important to identify novel non-apoptotic therapeutic agents. Several non-apoptotic programmed cell death pathways utilize specific cellular signaling events to trigger lytic and pro-inflammatory cell death. PANoptosis, which encompasses pyroptosis, apoptosis and necroptosis, is of paramount importance in the regulation of cell death and immune responses. Our study illustrates that ophiobolin A (OpA) is an anti-cancer agent that triggers lytic cell death in breast cancer cells, including triple-negative breast cancer (TNBC), via a mechanism dependent on RIPK1. This study reveals that OpA induces typical pyroptosis-like characteristics, including cellular swelling, plasma membrane rupture, GSDMD cleavage and release of cytokines in breast cancer cells. The involvement of caspase 3, RIPK1, and GSDMD suggests that PANoptosis is activated upon OpA treatment in breast cancer. The induction of pro-inflammatory cell death suggests potential applications for OpA in cancer treatment.
-
Cancer Research
-
Immunology and Microbiology
In Nature Communications on 9 April 2025 by Chen, Z. S., Peng, S. I., et al.
MicroRNAs (miRNAs) are small non-coding RNAs that play crucial roles in post-transcriptional gene regulation. Poly(A) RNA polymerase D5 (PAPD5) catalyzes the addition of adenosine to the 3' end of miRNAs. In this study, we demonstrate that the Yin Yang 1 protein, a transcriptional repressor of PAPD5, is recruited to both RNA foci and protein aggregates, resulting in an upregulation of PAPD5 expression in Huntington's disease (HD). Additionally, we identify a subset of PAPD5-regulated miRNAs with increased adenylation and reduced expression in our disease model. We focus on miR-7-5p and find that its reduction causes the activation of the TAB2-mediated TAK1-MKK4-JNK pro-apoptotic pathway. This pathway is also activated in induced pluripotent stem cell-derived striatal neurons and post-mortem striatal tissues isolated from HD patients. In addition, we discover that a small molecule PAPD5 inhibitor, BCH001, can mitigate cell death and neurodegeneration in our disease models. This study highlights the importance of PAPD5-mediated miRNA dysfunction in HD pathogenesis and suggests a potential therapeutic direction for the disease.
© 2025. The Author(s).
-
WB
-
Homo sapiens (Human)
In Journal of Virology on 17 September 2024 by BenDavid, E., Yang, C., et al.
Some negative-sense RNA viruses, including measles virus (MeV), share the characteristic that during their infection cycle, cytoplasmic inclusion bodies (IBs) are formed where components of the viral replication machinery are concentrated. As a foci of viral replication, how IBs act to enhance the efficiency of infection by affecting virus-host interactions remains an important topic of investigation. We previously established that upon MeV infection, the epigenetic host protein, WD repeat-containing protein 5 (WDR5), translocates to cytoplasmic viral IBs and facilitates MeV replication. We now show that WDR5 is recruited to IBs by forming a complex with IB-associated MeV phosphoprotein via a conserved binding motif located on the surface of WDR5. Furthermore, we provide evidence that WDR5 promotes viral replication by suppressing a major innate immune response pathway, the double-stranded RNA-mediated activation of protein kinase R and integrated stress response.
MeV is a pathogen that remains a global concern, with an estimated 9 million measles cases and 128,000 measles deaths in 2022 according to the World Health Organization. A large population of the world still has inadequate access to the effective vaccine against the exceptionally transmissible MeV. Measles disease is characterized by a high morbidity in children and in immunocompromised individuals. An important area of research for negative-sense RNA viruses, including MeV, is the characterization of the complex interactome between virus and host occurring at cytoplasmic IBs where viral replication occurs. Despite the progress made in understanding IB structures, little is known regarding the virus-host interactions within IBs and the role of these interactions in promoting viral replication and antagonizing host innate immunity. Herein we provide evidence suggesting a model by which MeV IBs utilize the host protein WDR5 to suppress the protein kinase R-integrated stress response pathway.
-
WB
-
Immunology and Microbiology
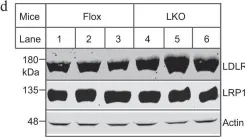
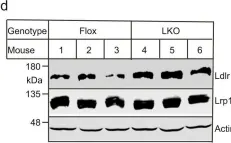
Oligodendrocyte-derived laminin-γ1 regulates the blood-brain barrier and CNS myelination in mice.
In Cell Reports on 28 May 2024 by Kang, M. & Yao, Y.
Although oligodendrocytes (OLs) synthesize laminin-γ1, the most widely used γ subunit, its functional significance in the CNS remains unknown. To answer this important question, we generated a conditional knockout mouse line with laminin-γ1 deficiency in OL lineage cells (γ1-OKO). γ1-OKO mice exhibit weakness/paralysis and die by post-natal day 33. Additionally, they develop blood-brain barrier (BBB) disruption in the cortex and striatum. Subsequent studies reveal decreased major facilitator superfamily domain containing 2a expression and increased endothelial caveolae vesicles, but unaltered tight junction protein expression and tight junction ultrastructure, indicating a transcellular, rather than a paracellular, mechanism of BBB breakdown. Furthermore, significantly reduced OL lineage cells, OL precursor cells (OPCs), proliferating OPCs, and mature OLs are observed in γ1-OKO brains in a region-specific manner. Consistent with this finding, various defects in myelination are detected in γ1-OKO brains at biochemical and ultrastructural levels. Overall, these results highlight important roles of OL-derived laminin-γ1 in BBB maintenance and OL biology (proliferation, differentiation, and myelination).
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
-
WB
-
Mus musculus (House mouse)
-
Cardiovascular biology
-
Neuroscience
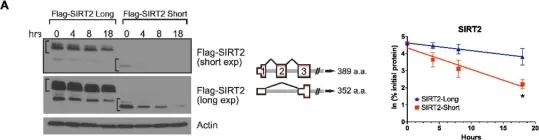
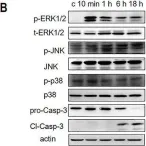
In Cells on 24 January 2024 by Chatterjee, B., Fatima, F., et al.
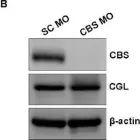
Fig.3.B

-
WB
-
Collected and cropped from Cells by CiteAb, provided under a CC-BY license
Image 1 of 38
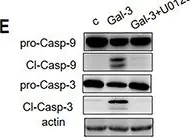
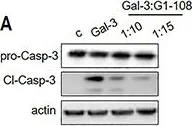
In Front Oncol on 3 January 2023 by Rai, Y., Singh, S., et al.
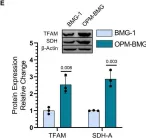
Fig.2.E

-
WB
-
Collected and cropped from Front Oncol by CiteAb, provided under a CC-BY license
Image 1 of 38
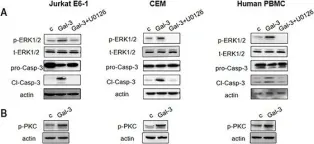
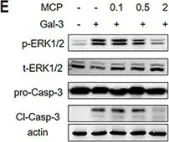
In Front Oncol on 3 January 2023 by Rai, Y., Singh, S., et al.
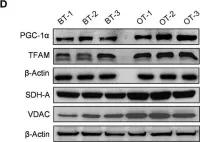
Fig.5.D

-
WB
-
Collected and cropped from Front Oncol by CiteAb, provided under a CC-BY license
Image 1 of 38
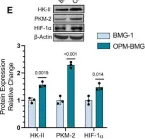
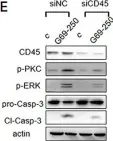
In Front Oncol on 3 January 2023 by Rai, Y., Singh, S., et al.
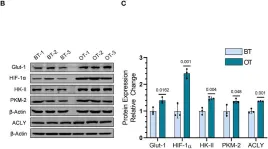
Fig.5.B

-
WB
-
Collected and cropped from Front Oncol by CiteAb, provided under a CC-BY license
Image 1 of 38
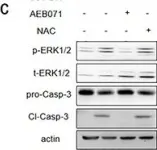
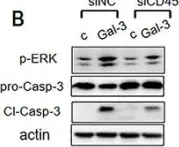
In Front Oncol on 3 January 2023 by Rai, Y., Singh, S., et al.
Fig.1.E

-
WB
-
Collected and cropped from Front Oncol by CiteAb, provided under a CC-BY license
Image 1 of 38
In Int J Mol Sci on 17 October 2022 by Cheng, S., Fahmi, N. A., et al.
Fig.4.A

-
WB
-
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 38
In Clin Transl Med on 1 August 2022 by Lee, J., Kim, S. J., et al.
Fig.3.B

-
WB
-
Collected and cropped from Clin Transl Med by CiteAb, provided under a CC-BY license
Image 1 of 38
In Clin Transl Med on 1 August 2022 by Lee, J., Kim, S. J., et al.
Fig.6.B

-
WB
-
Collected and cropped from Clin Transl Med by CiteAb, provided under a CC-BY license
Image 1 of 38
In Cell Death Dis on 22 April 2022 by Wang, B., Wu, H. H., et al.
Fig.5.E

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 38
In Cell Death Dis on 22 April 2022 by Wang, B., Wu, H. H., et al.
Fig.5.D

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 38
In Nat Commun on 25 March 2021 by Alabi, A., Xia, X. D., et al.
Fig.4.D

-
WB
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 38
In Nat Commun on 25 March 2021 by Alabi, A., Xia, X. D., et al.
Fig.5.D

-
WB
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 38
In Nat Commun on 25 March 2021 by Alabi, A., Xia, X. D., et al.
Fig.5.A

-
WB
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 38
In Nat Commun on 25 March 2021 by Alabi, A., Xia, X. D., et al.
Fig.2.C

-
WB
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 38
In Nat Commun on 25 March 2021 by Alabi, A., Xia, X. D., et al.
Fig.2.D

-
WB
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 38
In Sci Rep on 2 August 2017 by Sauvageau, E., McCormick, P. J., et al.
Fig.7.B

-
WB
-
Homo sapiens (Human)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 38
In Sci Rep on 2 August 2017 by Sauvageau, E., McCormick, P. J., et al.
Fig.9.B

-
WB
-
Homo sapiens (Human)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 38
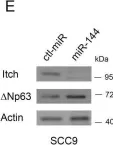
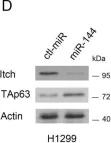
In Oncotarget on 25 July 2017 by Xue, H., Liu, L., et al.
Fig.1.B

-
WB
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 38
In Oncotarget on 25 July 2017 by Xue, H., Liu, L., et al.
Fig.2.E

-
WB
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 38
In Oncotarget on 25 July 2017 by Xue, H., Liu, L., et al.
Fig.4.A

-
WB
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 38
In Oncotarget on 25 July 2017 by Xue, H., Liu, L., et al.
Fig.6.C

-
WB
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 38
In Oncotarget on 25 July 2017 by Xue, H., Liu, L., et al.
Fig.7.A

-
WB
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 38
In Oncotarget on 25 July 2017 by Xue, H., Liu, L., et al.
Fig.7.E

-
WB
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 38
In Oncotarget on 25 July 2017 by Xue, H., Liu, L., et al.
Fig.8.E

-
WB
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 38
In Oncotarget on 25 July 2017 by Xue, H., Liu, L., et al.
Fig.8.B

-
WB
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 38