Mucosal-associated invariant T (MAIT) cells detect microbial infection via recognition of riboflavin-based antigens presented by the major histocompatibility complex class I (MHC-I)-related protein 1 (MR1). Most MAIT cells in human peripheral blood express CD8αα or CD8αβ coreceptors, and the binding site for CD8 on MHC-I molecules is relatively conserved in MR1. Yet, there is no direct evidence of CD8 interacting with MR1 or the functional consequences thereof. Similarly, the role of CD8αα in lymphocyte function remains ill-defined. Here, using newly developed MR1 tetramers, mutated at the CD8 binding site, and by determining the crystal structure of MR1-CD8αα, we show that CD8 engaged MR1, analogous to how it engages MHC-I molecules. CD8αα and CD8αβ enhanced MR1 binding and cytokine production by MAIT cells. Moreover, the CD8-MR1 interaction was critical for the recognition of folate-derived antigens by other MR1-reactive T cells. Together, our findings suggest that both CD8αα and CD8αβ act as functional coreceptors for MAIT and other MR1-reactive T cells.
© 2022 The University of Melbourne.
Product Citations: 5
In The Journal of Experimental Medicine on 5 September 2022 by Souter, M. N. T., Awad, W., et al.
-
FC/FACS
-
Rattus norvegicus (Rat)
-
Immunology and Microbiology
In Immunity on 10 May 2022 by Gideon, H. P., Hughes, T. K., et al.
Mycobacterium tuberculosis lung infection results in a complex multicellular structure: the granuloma. In some granulomas, immune activity promotes bacterial clearance, but in others, bacteria persist and grow. We identified correlates of bacterial control in cynomolgus macaque lung granulomas by co-registering longitudinal positron emission tomography and computed tomography imaging, single-cell RNA sequencing, and measures of bacterial clearance. Bacterial persistence occurred in granulomas enriched for mast, endothelial, fibroblast, and plasma cells, signaling amongst themselves via type 2 immunity and wound-healing pathways. Granulomas that drove bacterial control were characterized by cellular ecosystems enriched for type 1-type 17, stem-like, and cytotoxic T cells engaged in pro-inflammatory signaling networks involving diverse cell populations. Granulomas that arose later in infection displayed functional characteristics of restrictive granulomas and were more capable of killing Mtb. Our results define the complex multicellular ecosystems underlying (lack of) granuloma resolution and highlight host immune targets that can be leveraged to develop new vaccine and therapeutic strategies for TB.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
-
Immunology and Microbiology
In Cell Reports on 17 September 2019 by Hinks, T. S. C., Marchi, E., et al.
Mucosal-associated invariant T (MAIT) cells are MR1-restricted innate-like T cells conserved across mammalian species, including mice and humans. By sequencing RNA from sorted MR1-5-OP-RU tetramer+ cells derived from either human blood or murine lungs, we define the basic transcriptome of an activated MAIT cell in both species and demonstrate how this profile changes during the resolution of infection and during reinfection. We observe strong similarities between MAIT cells in humans and mice. In both species, activation leads to strong expression of pro-inflammatory cytokines and chemokines as well as a strong tissue repair signature, recently described in murine commensal-specific H2-M3-restricted T cells. Transcriptomes of MAIT cells and H2-M3-specific CD8+ T cells displayed the most similarities to invariant natural killer T (iNKT) cells when activated, but to γδ T cells after the resolution of infection. These data define the requirements for and consequences of MAIT cell activation, revealing a tissue repair phenotype expressed upon MAIT cell activation in both species.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
In Gastroenterology on 1 February 2019 by Uniken Venema, W. T., Voskuil, M. D., et al.
-
Homo sapiens (Human)
-
Cardiovascular biology
-
Genetics
-
Immunology and Microbiology
In PLoS Biology on 1 April 2014 by Sarrabayrouse, G., Bossard, C., et al.
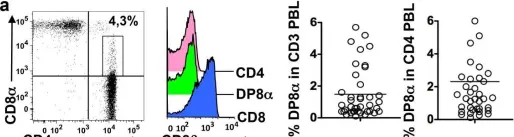
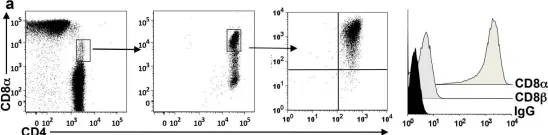
How the microbiota affects health and disease is a crucial question. In mice, gut Clostridium bacteria are potent inducers of colonic interleukin (IL)-10-producing Foxp3 regulatory T cells (Treg), which play key roles in the prevention of colitis and in systemic immunity. In humans, although gut microbiota dysbiosis is associated with immune disorders, the underlying mechanism remains unknown. In contrast with mice, the contribution of Foxp3 Treg in colitis prevention has been questioned, suggesting that other compensatory regulatory cells or mechanisms may exist. Here we addressed the regulatory role of the CD4CD8 T cells whose presence had been reported in the intestinal mucosa and blood. Using colonic lamina propria lymphocytes (LPL) and peripheral blood lymphocytes (PBL) from healthy individuals, and those with colon cancer and irritable bowel disease (IBD), we demonstrated that CD4CD8αα (DP8α) T lymphocytes expressed most of the regulatory markers and functions of Foxp3 Treg and secreted IL-10. Strikingly, DP8α LPL and PBL exhibited a highly skewed repertoire toward the recognition of Faecalibacterium prausnitzii, a major Clostridium species of the human gut microbiota, which is decreased in patients with IBD. Furthermore, the frequencies of DP8α PBL and colonic LPL were lower in patients with IBD than in healthy donors and in the healthy mucosa of patients with colon cancer, respectively. Moreover, PBL and LPL from most patients with active IBD failed to respond to F. prausnitzii in contrast to PBL and LPL from patients in remission and/or healthy donors. These data (i) uncover a Clostridium-specific IL-10-secreting Treg subset present in the human colonic LP and blood, (ii) identify F. prausnitzii as a major inducer of these Treg, (iii) argue that these cells contribute to the control or prevention of colitis, opening new diagnostic and therapeutic strategies for IBD, and (iv) provide new tools to address the systemic impact of both these Treg and the intestinal microbiota on the human immune homeostasis.
-
FC/FACS
-
Immunology and Microbiology
In PLoS Biol on 1 April 2014 by Sarrabayrouse, G., Bossard, C., et al.
Fig.5.A

-
FC/FACS
-
Collected and cropped from PLoS Biol by CiteAb, provided under a CC-BY license
Image 1 of 2
In PLoS Biol on 1 April 2014 by Sarrabayrouse, G., Bossard, C., et al.
Fig.6.A

-
FC/FACS
-
Collected and cropped from PLoS Biol by CiteAb, provided under a CC-BY license
Image 1 of 2