Abstract Mutations in NLRP3 cause a spectrum of autoinflammatory disease, cryopyrin-associated periodic syndromes (CAPS). Reactive oxygen species (ROS) play a key role in NLRP3 inflammasome activation. We identified cofilin-1, an actin-severing protein, as a negative regulator of the NLRP3 inflammasome. In resting cells, cofilin-1 directly bound NLRP3, but upon stimulation with NLRP3 inflammasome activators, it was oxidized by ROS and dissociated from NLRP3. CAPS-associated mutant NLRP3 exhibited reduced binding to cofilin-1 compared to wild-type. Residues 101–104 of cofilin-1 were critical for NLRP3 interaction. Oxidation-resistant peptides containing this NLRP3-binding motif suppressed inflammasome activation induced by endogenous CAPS-associated mutations and ex vivo NLRP3 activators such as ATP and nigericin, but not flagellin. Bioinformatic structural analyses corroborate a model in which cofilin-1 plays a pivotal role in NLRP3 activation by ROS and support the potential use of cofilin-1-derived peptides in patients who are unresponsive to or intolerant of other forms of NLRP3 blockade.
Cofilin-1 is a Redox-Sensitive Guard of the NLRP3 Inflammasome
Preprint on Research Square on 23 May 2025 by Chae, J. J., Park, Y. H., et al.
In Journal of Translational Medicine on 23 December 2024 by Lai, Y., Zhuang, L., et al.
Renal CD8+ tissue-resident memory T (TRM) cells display prolonged survival and activity in lupus nephritis (LN), exacerbating renal pathology. NLRP3 regulates the T cell response. This study explored the impact of NLRP3 inflammasome activity on the regulatory functions of TRM cells in LN.
NLRP3 inflammasome activity in renal CD8+ TRM cells from lupus-prone MRL/lpr mice and in vitro induced human CD8+CD103+ T cells was assessed by quantifying NLRP3, caspase-1, gasdermin D (GSDMD), and IL-1β levels using flow cytometry, ELISA, and western blotting analysis. The specific NLRP3 inhibitor MCC950, caspase-1 inhibitor Ac-YVAD-cmk, and NF-κB inhibitor JSH23 were utilized to delineate the role of NLRP3 in modulating the pathogenicity of CD8+ TRM cells in LN.
Activation of the NLRP3 inflammasome was confirmed in renal CD8+CD69+CD103+ TRM cells derived from mice with LN and in vitro-induced human CD8+CD103+ TRM-like cells. MCC950 curtailed the infiltration and activity of CD8+CD69+CD103+ TRM cells and enhanced renal outcomes. MCC950 also suppressed the maturation and functional capabilities of CD8+CD103+ T cells in a manner reliant on inflammasome activity in vitro. IL-1β promoted the expression of TGF-βRII in CD8+ T cells via the NF-κB pathway.
NLRP3 inflammasome activity in renal CD8+CD69+CD103+ TRM cells contributes to LN pathogenesis by regulating cell differentiation and effector functions. Therapeutically targeting the NLRP3 inflammasome could significantly mitigate CD8+CD69+CD103+ TRM cell-mediated renal damage in LN.
© 2024. The Author(s).
In BMC Oral Health on 19 December 2024 by Huang, M., Yang, B., et al.
IFN-γ is crucial in induction of inducible cell-autonomous immunity, and IFN-γ signaling pathway is activated in pulpitis. Guanylate-binding proteins (GBPs) are a family of IFN-inducible GTPases and could utilize autophagy or pyroptosis to mitigate infection. GBP5 is abundantly expressed in inflamed pulp and human dental pulp cells (HDPCs). Therefore, we hypothesize that GBP5 in HDPCs exerts an immune-regulatory role in defending against bacterium infection.
Fusobacterium nucleatum (F. nucleatum) was used to infect HDPCs, and immunoblotting and qRT-PCR were used to detect pyroptosis and autophagy. Pharmacological or genetic approaches were used to enhance or knock down GBP5 expression in HDPCs. Blood agar plate counting and immunoblotting were used to observe bacteria clearance effect and activation of autophagy. Student's t-test and one-way ANOVA were individually used for comparisons between two and multiple groups. Statistical significance was set at P < 0.05.
Following F. nucleatum infection in HDPCs, the autophagy marker LC3B was significantly upregulated while the mRNA and protein expression levels of p62 were increased. IFN-γ priming significantly inhibited the intracellular survival of F. nucleatum and enhanced the autophagic activity of HDPCs. GBP5 overexpression significantly increased the efficiency of HDPCs in clearing intracellular F. nucleatum and activated autophagic flux in HDPCs, while downregulating GBP5 in HDPCs suppressed autophagic flux.
IFN-γ-mediated GBP5 overexpression in HDPCs during F. nucleatum infection exerts an anti-microbial function through autophagy activation.
© 2024. The Author(s).
Comparison of Different Keratinocyte Cell Line Models for Analysis of NLRP1 Inflammasome Activation.
In Biomolecules on 8 November 2024 by Wang, T., Yazdi, A. S., et al.
The NLRP1 (nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-1) inflammasome is the most important inflammasome in human keratinocytes. It plays a crucial role in regulating innate immunity in the skin. This study aimed to evaluate NLRP1 inflammasome activation and the corresponding levels of detection in different keratinocyte cell lines to identify a suitable in vitro model for analyzing inflammasome activation in keratinocytes. We compared NLRP1 inflammasome activation, expression, and cell death among primary keratinocytes and immortalized keratinocyte cell lines HaCaT, HaSKpw, and SVTERT upon stimulation with ultraviolet B (UVB) irradiation or talabostat. The effects of both NLRP1 inducers on cell death and the modification of NLRP1 molecules were examined using fluorescence-activated cell sorting analysis, Western blotting, and an enzyme-linked immunosorbent assay. The key inflammasome components had varied expression levels among the keratinocyte cell models, with the highest expression observed in primary keratinocytes. Moreover, our data showed that both UVB and talabostat triggered cell death, and NLRP1 inflammasome activation was readily detected in primary keratinocytes but not in the analyzed immortalized keratinocyte cell lines. Therefore, we do not recommend the use of the immortalized keratinocyte cell lines HaCaT, HaSKpw, and SVTERT for analyzing inflammasome activation in keratinocytes; we strongly recommend the use of primary keratinocytes for these studies.
In Journal of Innate Immunity on 5 November 2024 by Bettoni, S., Dziedzic, M., et al.
Streptococcus pyogenes (group A streptococcus; GAS) is a pathogen causing over half a million deaths annually worldwide. Human immune cells respond to GAS infection by activating the NLRP3 inflammasome leading to the release of pro-inflammatory cytokines that control infection. We investigated the role of C4b-binding protein (C4BP) and factor H (FH) in the inflammasome response to GAS, as they are recruited by GAS to prevent complement deposition and limit phagocytosis.
The inflammasome response was investigated using primary human cells and the strain GAS-AP1. Cytokine responses were evaluated by ELISA. C4BP internalisation was investigated using confocal microscopy. Activation of the NLRP3 inflammasome components was assessed by Western blotting.
Interleukin-1β (IL-1β) release, induced by GAS-AP1, was inhibited by FH which interferes with priming of human cells. In contrast, C4BP restricted the IL-1β response without affecting cell priming. C4BP was engulfed by cells together with bacteria and excluded from low-pH vesicles but localised within the cytosol and near the ASC speck inflammasome complex. C4BP did not inhibit either the inflammasome complex assembly or caspase-1 activation. However, C4BP limited the cleavage of gasdermin D N-terminal fragments by interfering with caspase-1 enzymatic activity.
Given that the amount of IL-1β modulates the severity of GAS infection, our results provide new insights into the effect of FH and internalised C4BP to control GAS sensing by inflammasomes.
© 2024 The Author(s). Published by S. Karger AG, Basel.
In J Transl Med on 23 December 2024 by Lai, Y., Zhuang, L., et al.
Fig.2.H
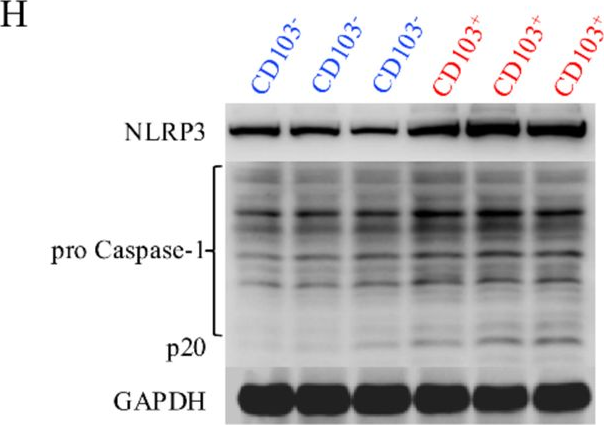
-
WB
-
Collected and cropped from Journal of Translational Medicine by CiteAb, provided under a CC-BY license
Image 1 of 12
In J Transl Med on 23 December 2024 by Lai, Y., Zhuang, L., et al.
Fig.3.E
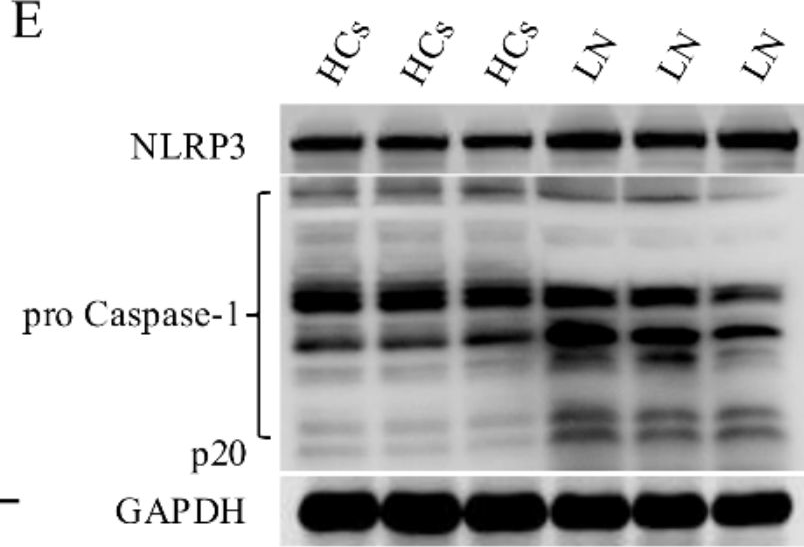
-
WB
-
Collected and cropped from Journal of Translational Medicine by CiteAb, provided under a CC-BY license
Image 1 of 12
In Cell Rep on 23 April 2019 by Goddard, P. J., Sanchez-Garrido, J., et al.
Fig.1.C
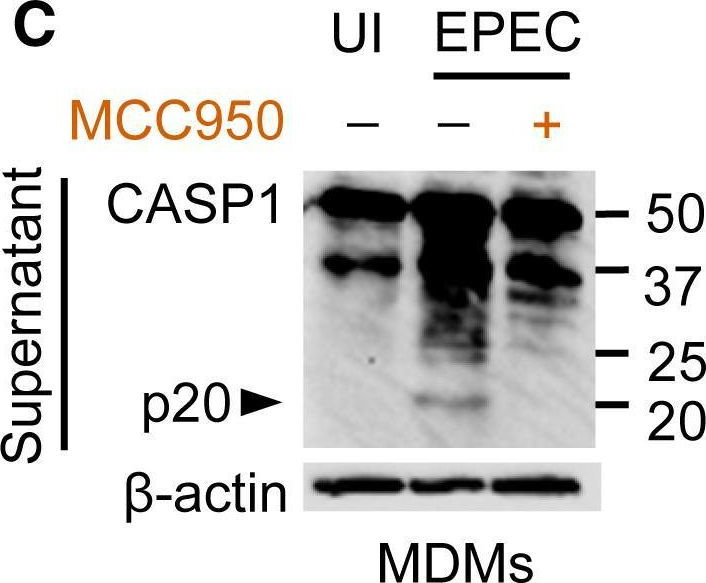
-
WB
-
Homo sapiens (Human)
Collected and cropped from Cell Reports by CiteAb, provided under a CC-BY license
Image 1 of 12
In Cell Rep on 23 April 2019 by Goddard, P. J., Sanchez-Garrido, J., et al.
Fig.1.F
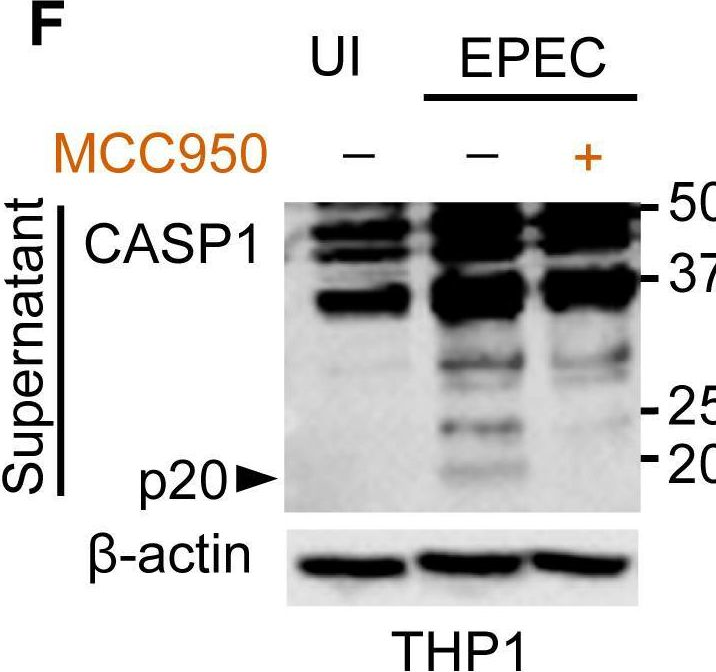
-
WB
-
Homo sapiens (Human)
Collected and cropped from Cell Reports by CiteAb, provided under a CC-BY license
Image 1 of 12
In Cell Rep on 23 April 2019 by Goddard, P. J., Sanchez-Garrido, J., et al.
Fig.2.C
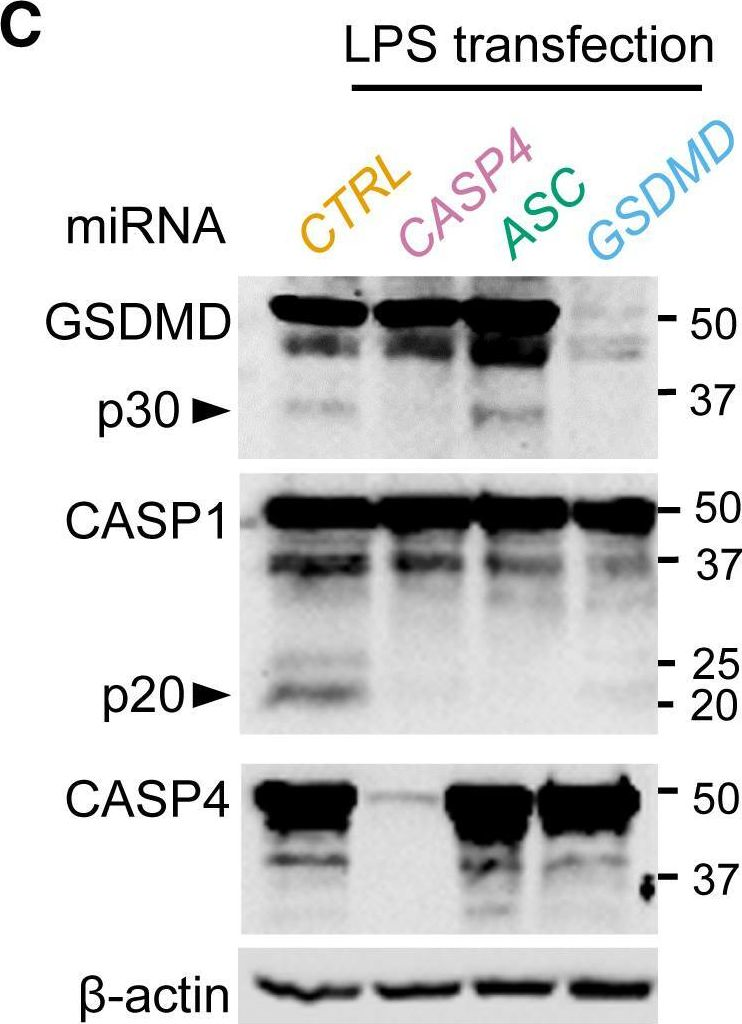
-
WB
-
Homo sapiens (Human)
Collected and cropped from Cell Reports by CiteAb, provided under a CC-BY license
Image 1 of 12
In Cell Rep on 23 April 2019 by Goddard, P. J., Sanchez-Garrido, J., et al.
Fig.2.D
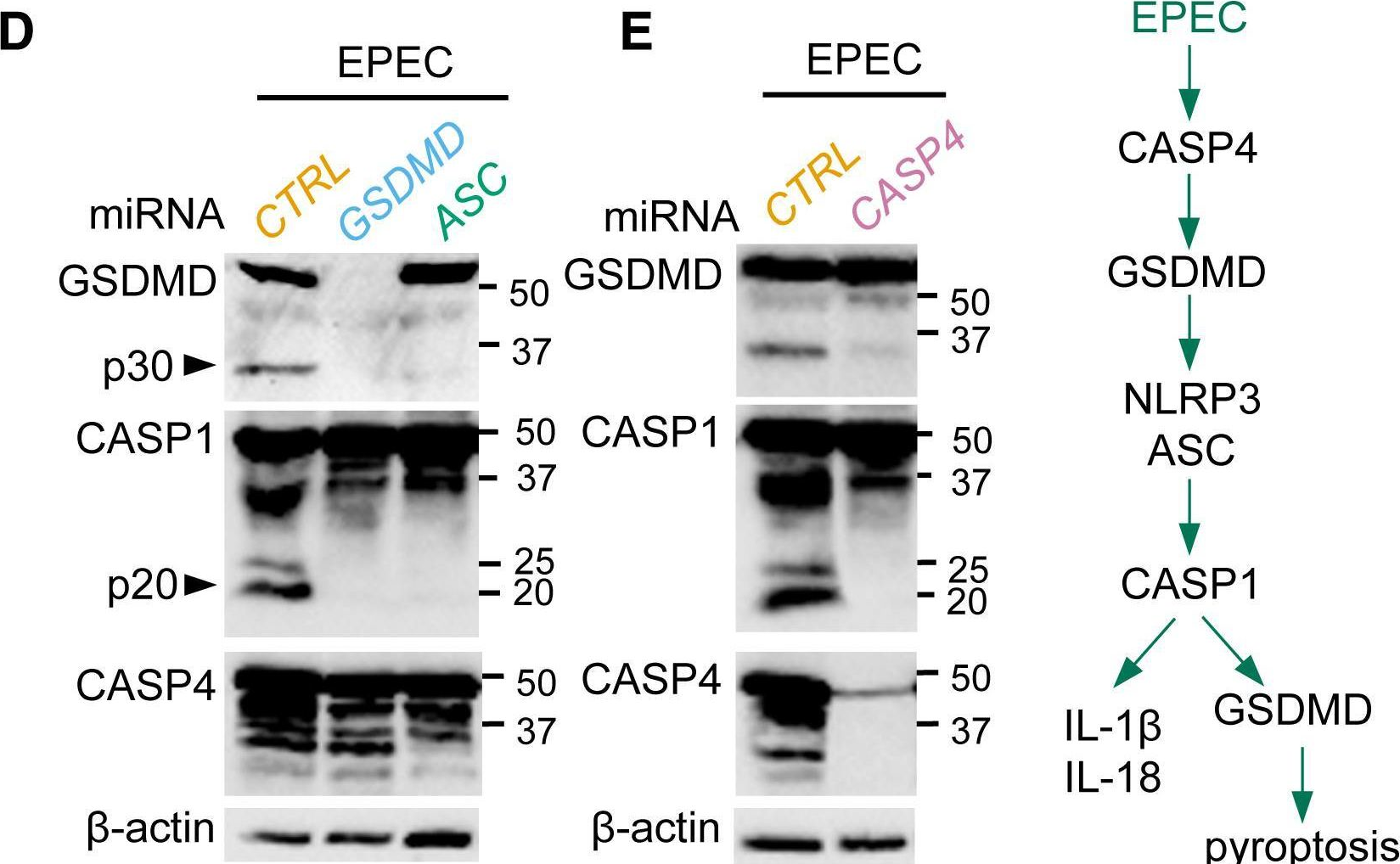
-
WB
-
Homo sapiens (Human)
Collected and cropped from Cell Reports by CiteAb, provided under a CC-BY license
Image 1 of 12
In Cell Rep on 23 April 2019 by Goddard, P. J., Sanchez-Garrido, J., et al.
Fig.4.B
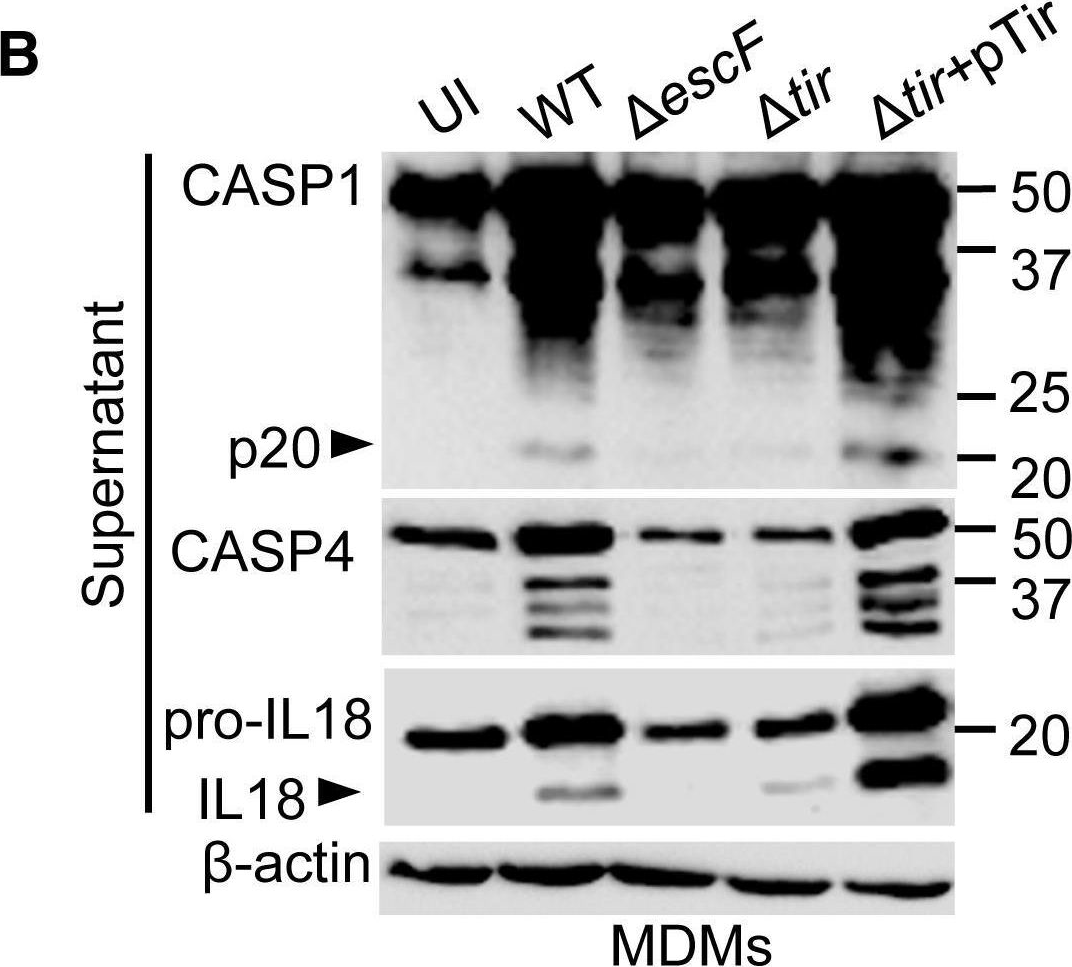
-
WB
-
Homo sapiens (Human)
Collected and cropped from Cell Reports by CiteAb, provided under a CC-BY license
Image 1 of 12
In Cell Rep on 23 April 2019 by Goddard, P. J., Sanchez-Garrido, J., et al.
Fig.5.G
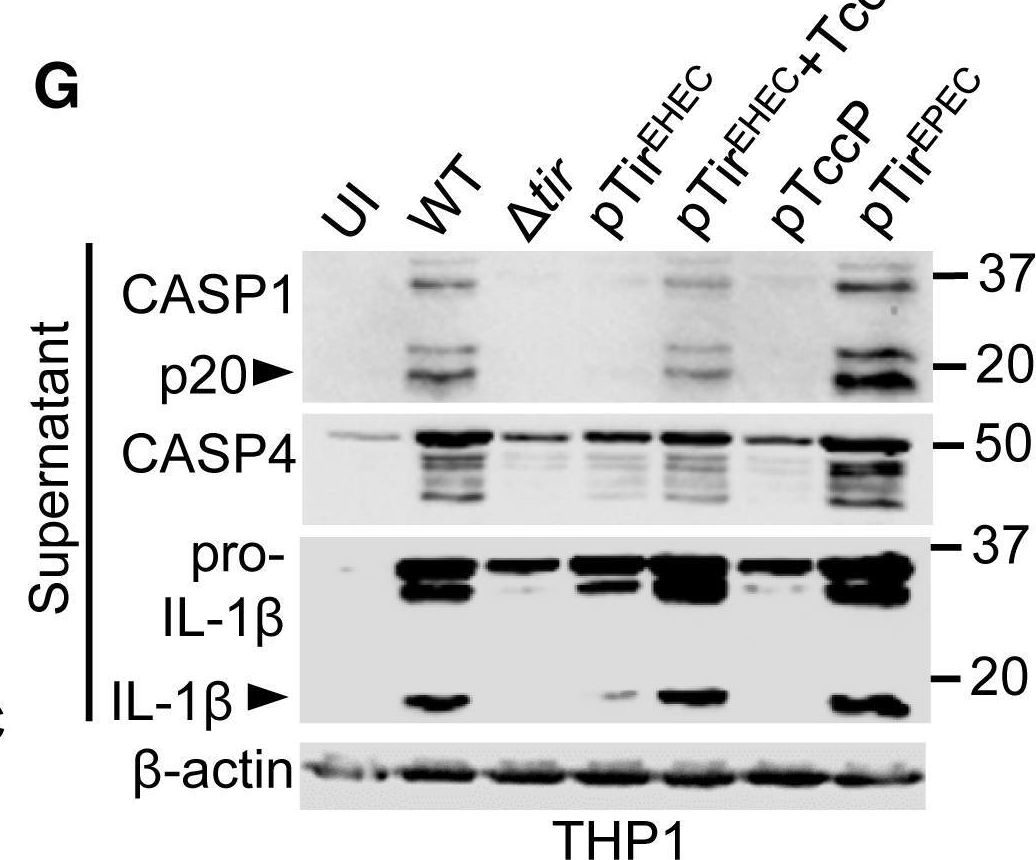
-
WB
-
Homo sapiens (Human)
Collected and cropped from Cell Reports by CiteAb, provided under a CC-BY license
Image 1 of 12
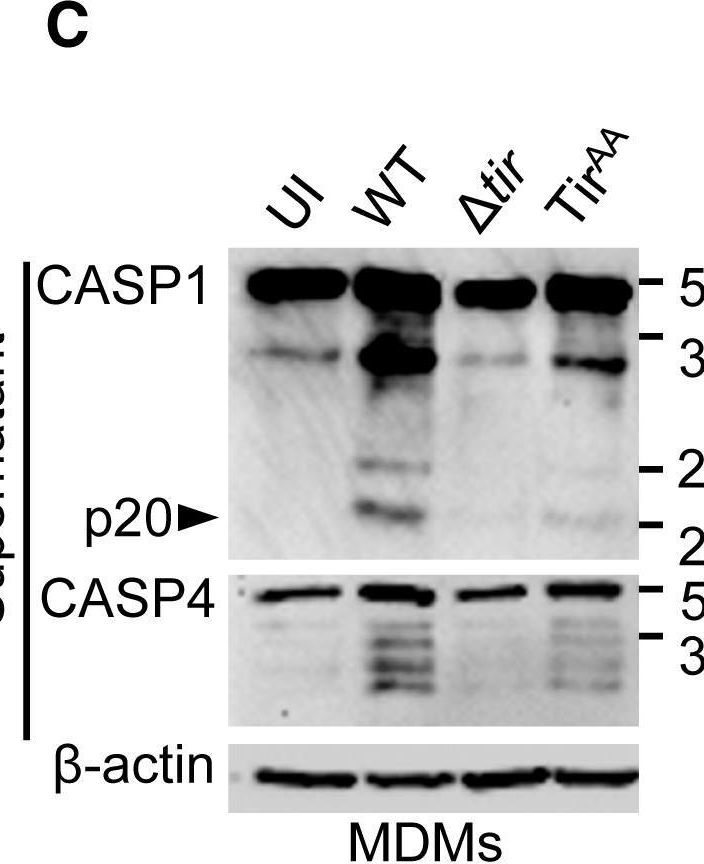
In Cell Rep on 23 April 2019 by Goddard, P. J., Sanchez-Garrido, J., et al.
Fig.5.C
-
WB
-
Homo sapiens (Human)
Collected and cropped from Cell Reports by CiteAb, provided under a CC-BY license
Image 1 of 12
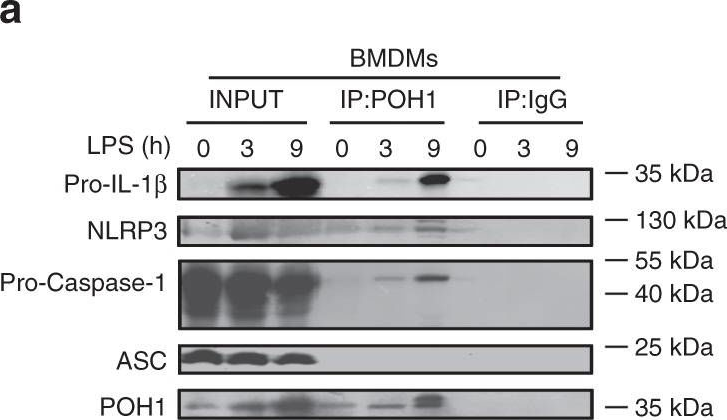
In Nat Commun on 12 October 2018 by Zhang, L., Liu, Y., et al.
Fig.5.A
-
WB
-
Mus musculus (House mouse)
Collected and cropped from Nature Communications by CiteAb, provided under a CC-BY license
Image 1 of 12
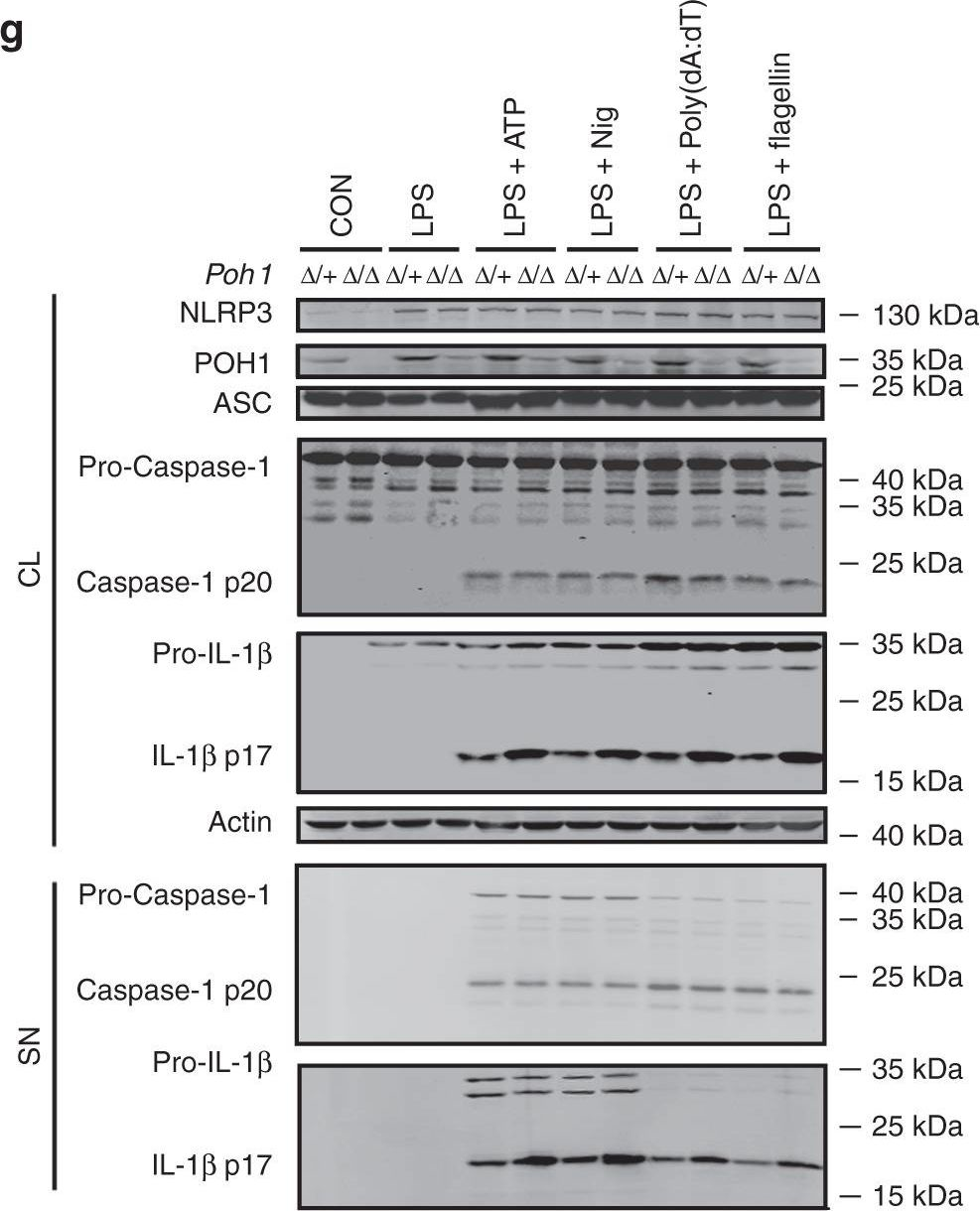
In Nat Commun on 12 October 2018 by Zhang, L., Liu, Y., et al.
Fig.3.G
-
WB
-
Mus musculus (House mouse)
Collected and cropped from Nature Communications by CiteAb, provided under a CC-BY license
Image 1 of 12
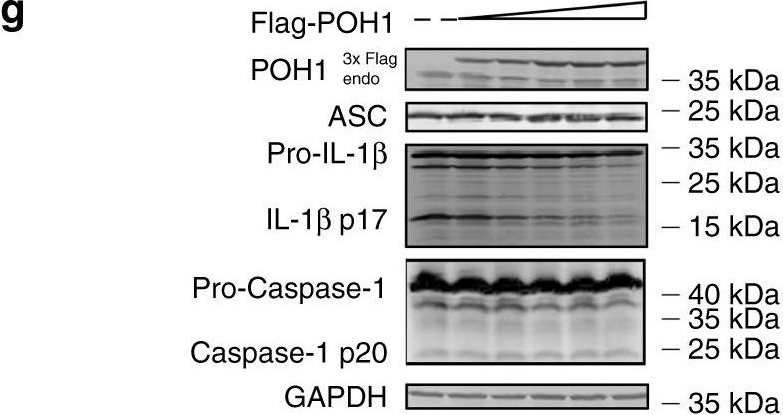
In Nat Commun on 12 October 2018 by Zhang, L., Liu, Y., et al.
Fig.4.G
-
WB
-
Mus musculus (House mouse)
Collected and cropped from Nature Communications by CiteAb, provided under a CC-BY license
Image 1 of 12
